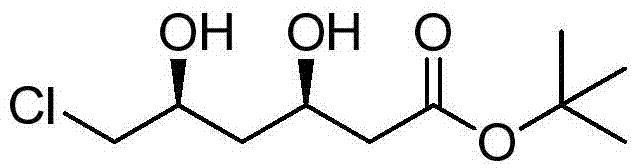
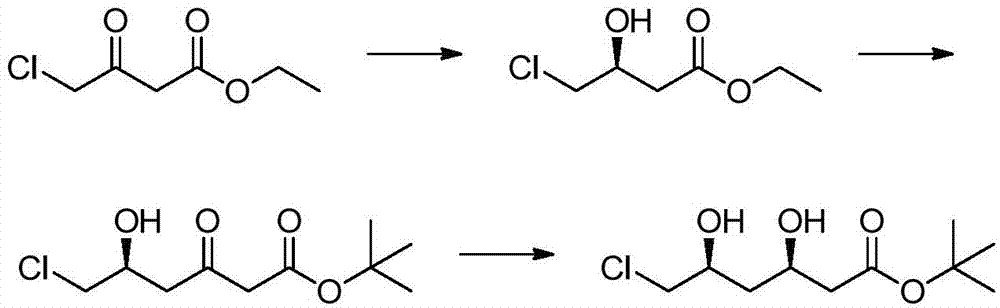
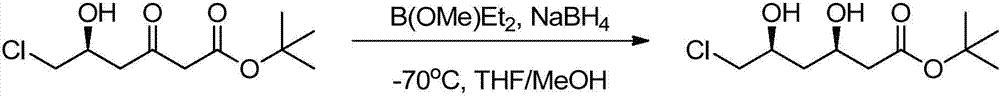Preparation method of tert-butyl (3R,5S)-6-chloro-3,5-dihydroxyhexanoate
A technology of tert-butyl hydroxyhexanoate and tert-butyl carbonylhexanoate, which is applied in the field of preparation of (3R,5S)-6-chloro-3,5-dihydroxyhexanoate tert-butyl, can solve the problem of large enzyme consumption , Low substrate concentration, low product yield and other problems, to achieve the effect of increasing substrate concentration, increasing substrate concentration, and reducing the amount of enzyme
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Substrate (S)-150 g of 6-chloro-5-hydroxy-3-oxoylhexanoic acid tert-butyl ester and 252 g of glucose were added to a 2 L reactor filled with 0.3 L of 50 mM pH 6.5 phosphate buffer solution, Add 3.75 g of KRED (purchased from Suzhou Hanzyme, brand EW005), 1.25 g of GDH (purchased from Suzhou Hanzyme, brand EW002), 0.75 g of mercaptoethanol, and 0.3 g of NADP, and control with 30% sodium carbonate solution at 30°C PH6.2, stirred for 24 hours, HPLC / MS detected that the reaction was complete, added an equal volume of ethyl acetate to filter, took the organic phase, extracted the aqueous phase twice with an equal volume of ethyl acetate, and combined the organic phases to obtain the product (3R, 5S )-6-chloro-3,5-dihydroxyhexanoic acid tert-butyl ester 145 g of light yellow liquid, de value 99.9%, purity 99.0%.
Embodiment 2
[0032] Substrate (S)-150 g of 6-chloro-5-hydroxy-3-oxoylhexanoic acid tert-butyl ester and 252 g of glucose were added to a 2 L reactor filled with 0.3 L of 50 mM pH 6.5 phosphate buffer solution, Add 3.75 g of KRED (purchased from Suzhou Hanzyme, brand EW005), 1.25 g of GDH (purchased from Suzhou Hanzyme, brand EW002), 0.75 g of mercaptoethanol, and 0.3 g of NADP, and control with 20% sodium carbonate solution at 30 °C PH6.3, stirred for 24 hours, HPLC / MS detected that the reaction was complete, added an equal volume of ethyl acetate to filter, took the organic phase, extracted the aqueous phase twice with an equal volume of ethyl acetate, combined the organic phases and rotary evaporated to obtain the product (3R, 5S )-6-chloro-3,5-dihydroxyhexanoic acid tert-butyl ester 145 g of light yellow liquid, de value 99.9%, purity 99.0%.
Embodiment 3
[0034] Substrate (S)-150 g of 6-chloro-5-hydroxy-3-oxoylhexanoic acid tert-butyl ester and 252 g of glucose were added to a 2 L reactor filled with 0.3 L of 50 mM pH 6.5 phosphate buffer solution, Add 3.75 g of KRED (purchased from Suzhou Hanzyme, brand EW005), 1.25 g of GDH (purchased from Suzhou Hanzyme, brand EW002), 0.75 g of mercaptoethanol, and 0.3 g of NADP, and control with 10% sodium carbonate solution at 30°C PH6.2, stirred for 24 hours, HPLC / MS detected that the reaction was complete, added an equal volume of ethyl acetate to filter, took the organic phase, extracted the aqueous phase twice with an equal volume of ethyl acetate, and combined the organic phases to obtain the product (3R, 5S )-6-chloro-3,5-dihydroxyhexanoic acid tert-butyl ester 145 g of light yellow liquid, de value 99.9%, purity 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com