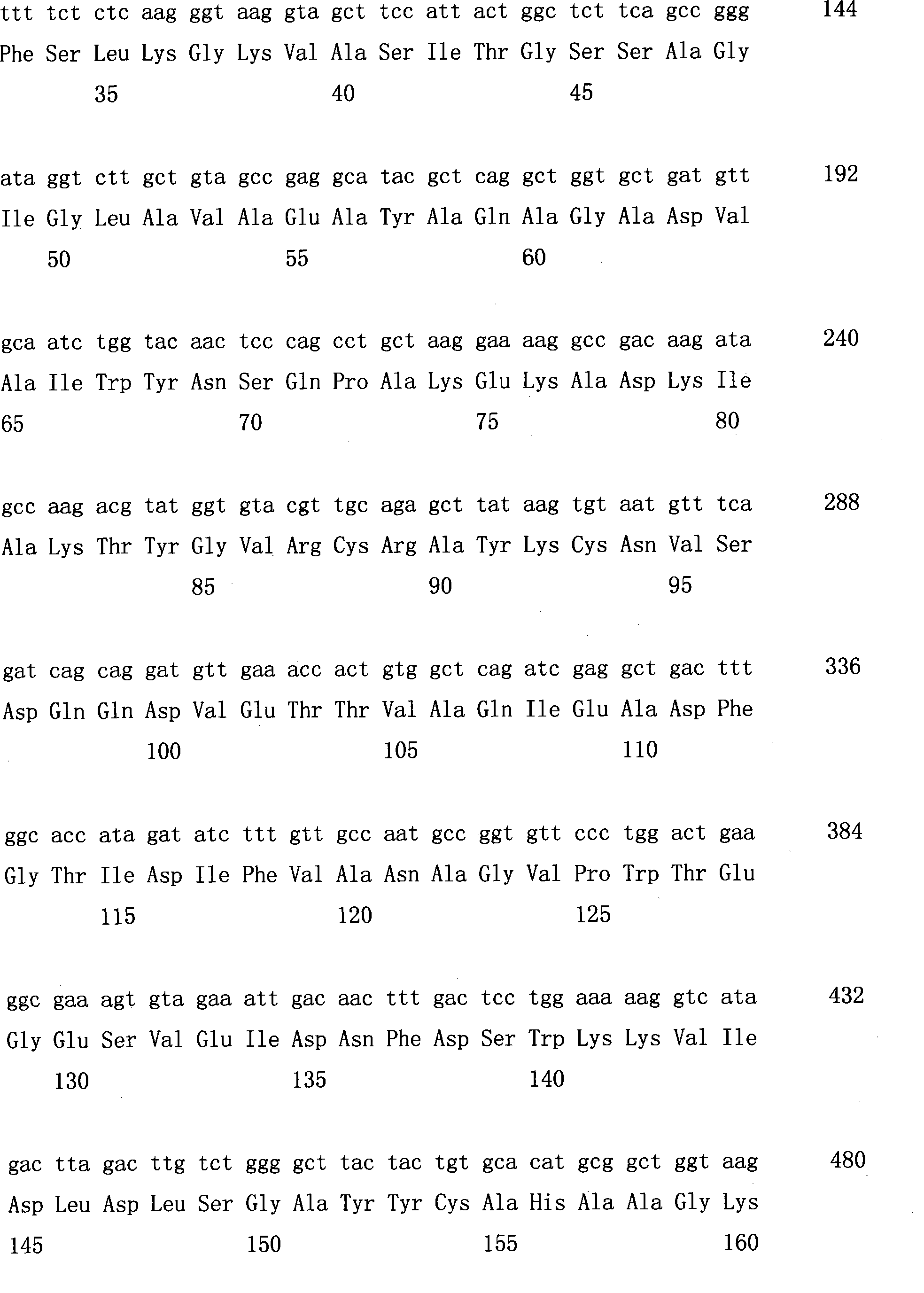Recombinant yeast for unsymmetrical conversion and preparation of (S)-4-chloro-3-hydroxybutanoate and construction method and use thereof
A technology of ethyl hydroxybutyrate and ethyl chloroacetoacetate, which is applied in the field of biocatalytic asymmetric transformation, can solve the problems of difficulty in screening microbial strains and low optical activity of products, achieve high forwarding rate, high optical activity, and reduce production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
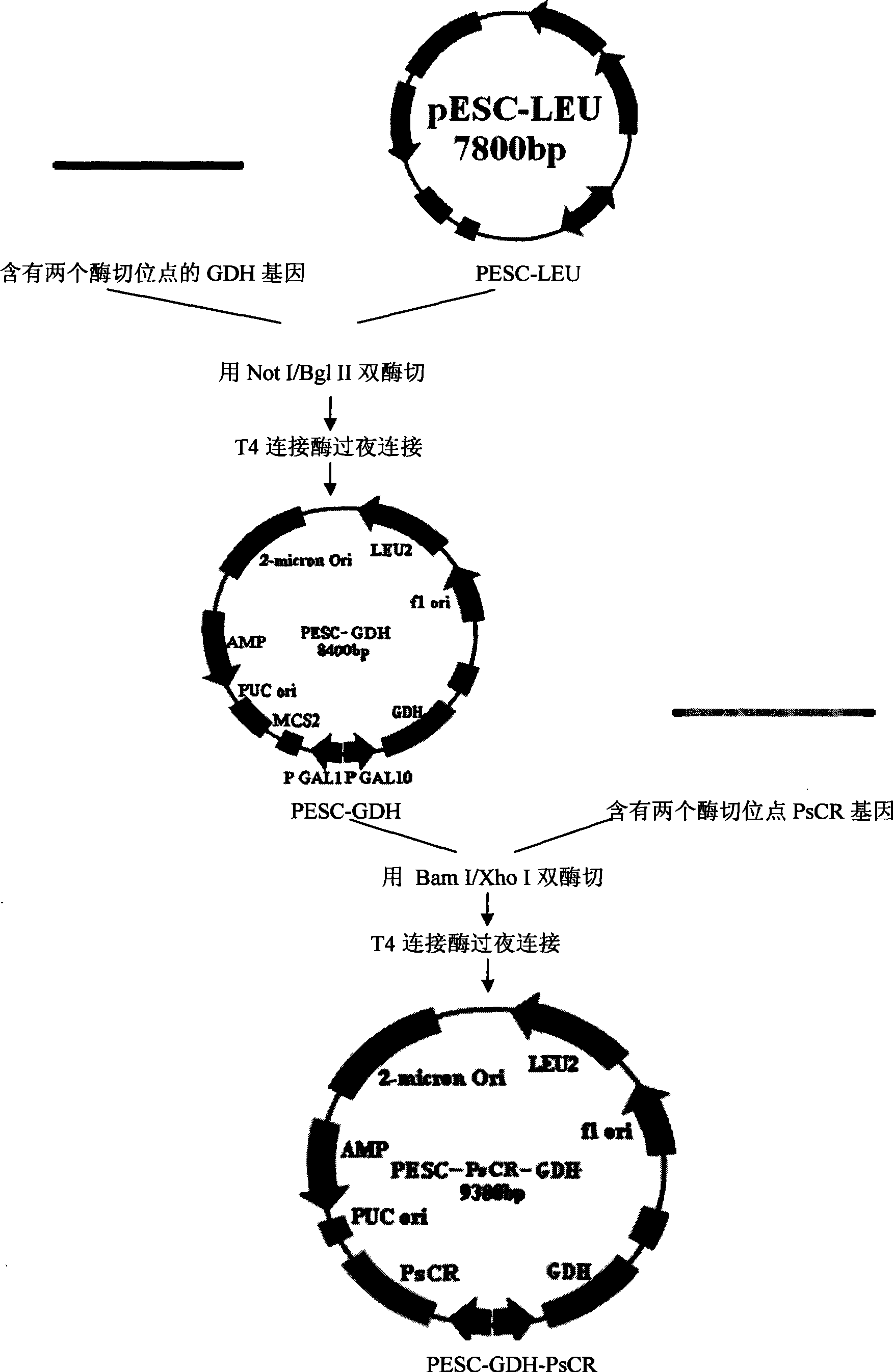
[0038] Embodiment 1: Construction of recombinant yeast
[0039] 1. Acquisition of carbonyl reductase PsCR gene and glucose dehydrogenase GDH gene.
[0040] Pichia stipitis CBS 6054 medium YPD (g L -1 ): Yeast extract 10g, peptone 20g, glucose 20g.
[0041] Pichia pastoris was inoculated in 5 mL of YPD liquid medium and cultured at 30°C until the logarithmic growth phase, and the genome was extracted using a genomic DNA extraction kit (kit from Beijing Tianwei Bioengineering Co., Ltd.).
[0042] The primers used to construct MCS1 in the expression vector are added with restriction sites, and the primer sequences are as follows:
[0043] The upstream primer (-sense contains BamH I) is: 5'CGC GGATCC ATGGCTAAGAACTTCTCCAAC
[0044] The downstream primer (-anti containing Xho I) is: CCG CTCGAG TTAGGGAAGCGTGTAGCCAC
[0045] All primers were synthesized by Shanghai Shenergy Gaming Company.
[0046] Gene PCR conditions (50μL system):
[0047] Denaturation at 94°C for 7 min, f...
Embodiment 2
[0082] Embodiment 2: Fermentation of recombinant yeast (CCTCC NO: M 208129)
[0083] Pick the monoclonal PESC-GDH-PsCR that has been identified as positive, and inoculate it in 5 mL of leucine-deficient SD medium, overnight at 30°C. Inoculate in leucine-deficient SG medium according to the inoculum amount of 2%, and induce for 16 hours at 30°C. 8000rpm, centrifuge at 4°C for 10min, discard the supernatant.
[0084] The formula of SG medium is as follows:
[0085] Galactose 20g
[0086] Yeast nitrogen source (not containing leucine) 6.7g, its formula is shown in Table 1;
[0087] Water to 1000ml. Example 3:
Embodiment 3
[0088] The precipitate of Example 2 was washed twice with potassium phosphate buffer (100mmol·L-1, pH 6.0), weighed 0.5g (wet weight) of recombinant yeast sludge, and suspended in 5mL of pH 6.0 potassium phosphate buffer. Cells were sonicated (power 300W, sonication 5s, intermittent 5s, 5min in total), adding glucose 100mmol / L, COBE 1g / L, 24°C, 190rpm, 18h. The yield of the product (S)-CHBE is 0.99 g / L, the yield of the product is: 99%, and the optical purity e.e% is 100%.
[0089] The detection method of (S)-CHBE is as follows, and the detection method of product is identical in the following examples:
[0090] For the aqueous phase reaction: after the reaction, add an equal volume of ethyl acetate, vibrate vigorously for 10 minutes, then place it for two hours, and centrifuge at 8000rpm for 10 minutes to separate the organic layer and the aqueous layer. Carefully draw the upper layer of ethyl acetate through the organic membrane, add the internal standard, and save the test...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com