Method for preparing (R)-2-hydroxy-4-phenyl ethyl butyrate by catalyzing with recombinant carbonyl reductase
A technology of ethyl phenylbutyrate and carbonyl reductase, applied in the field of bioengineering, can solve problems such as expensive reagents, high price, harsh reaction conditions, etc., and achieve the effects of improving conversion effect, solving regeneration problem, and solving inhibition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Acquisition of carbonyl reductase gene
[0032] Bacillus subtilis ( Bacillus subtilis ) CGMCC NO.1.1508 comes from China General Microorganism Culture Collection and Management Center, medium LB (g / L): 10 g tryptone, 5 g yeast extract, 10 g sodium chloride, add water to 1 L. Bacillus subtilis was inoculated in 50 mL of liquid medium and cultured at 37 °C to the logarithmic phase, and the genome was extracted using a genomic DNA extraction kit. The primer sequences were designed as follows:
[0033] The upstream primer IolSf contains N de I
[0034] 5'-GGAATTC CATATG AAAAAAAGCGAAGCTCGG-3'
[0035] The downstream primer IolSr contains X ho I
[0036] 5'-CCG CTCGAG TGCGAACAGCTTATCAAT-3'
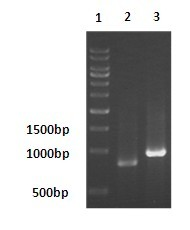
[0037] PCR conditions were: denaturation at 95°C for 5 min, followed by 30 cycles with the following parameters: denaturation at 95°C for 1 min, annealing at 62°C for 50 s, and extension at 72°C for 1 min. A final extension at 72°C for 10 min. PCR results such as ...
Embodiment 2
[0038] Example 2 Expression of carbonyl reductase gene
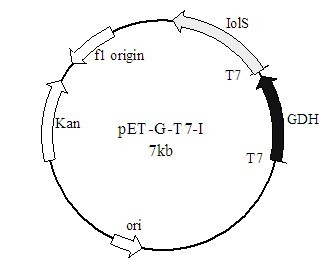
[0039] use N de I and X pET20b and carbonyl reductase genes were digested with ho I respectively, and the recovered products were ligated overnight with T4 ligase. The ligation product was added to Escherichia coli BL21(DE3) competent cells, transformed by heat shock method, spread on LB plates containing 100 μg / mL ampicillin, and incubated at 37°C for 12 h. Positive transformants were selected by ampicillin resistance carried by the vector. Positive recombinants were verified by colony PCR and extracted plasmid double enzyme digestion.
Embodiment 3
[0040] Example 3 Acquisition of Glucose Dehydrogenase Gene
[0041] Strain cultivation and gene extraction were as in Example 1. The primer sequences were designed as follows:
[0042] The upstream primer GDHf contains N de I
[0043] 5'-GGAATTC CATATG TATCCGGATTTAAAAGC-3'
[0044] The downstream primer GDHr contains H ind III
[0045] 5'-CCC AAGCTT TTAACCGCGGCCTGC-3'
[0046] PCR conditions were: denaturation at 95°C for 5 min, followed by 30 cycles with the following parameters: denaturation at 95°C for 1 min, annealing at 55°C for 50 s, and extension at 72°C for 1 min. Finally, extend at 72°C for 10 min. PCR results such as figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com