Alcohol dehydrogenase, gene and recombinase thereof, and application of alcohol dehydrogenase in synthesis of chiral diaryl secondary alcohol
An alcohol dehydrogenase and gene-encoding technology, which can be used in applications, genetic engineering, plant genetic improvement, etc., can solve the problems of low enzyme yield, low overall catalytic activity, and narrow asymmetric reduction substrates, and achieve atom economy. High, environmentally friendly, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
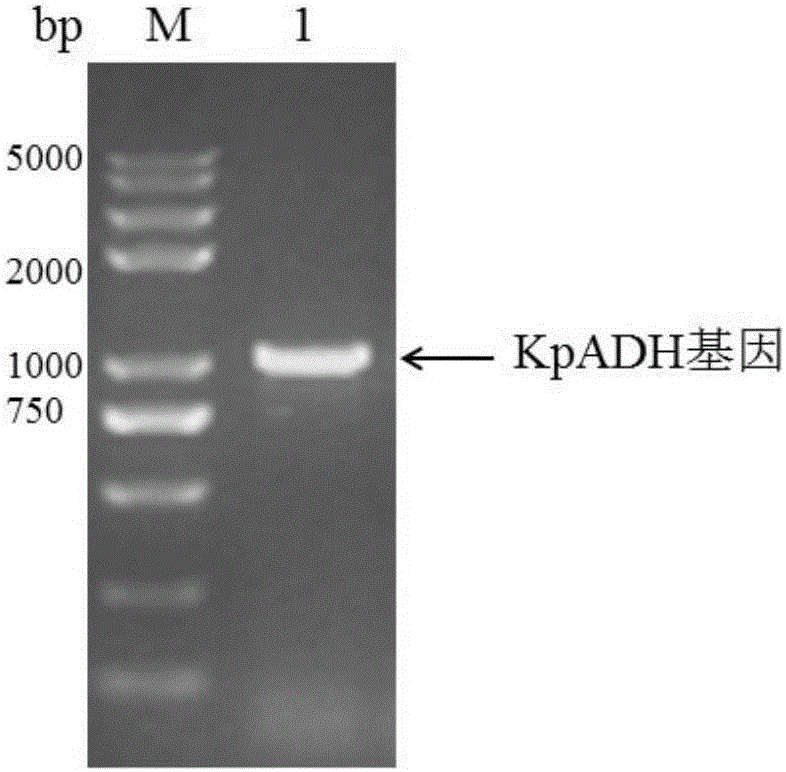
[0044] Cloning of embodiment 1 alcohol dehydrogenase gene
[0045] The above Kluyveromyces alcohol dehydrogenase gene was cloned by shotgun method.
[0046] (1) First, the above-mentioned Kluyveromyces sp. CCTCCM2011385 was cultured overnight at 37° C. using LB medium. After centrifugation to obtain the bacterial cells, use a conventional yeast genome extraction kit to obtain total genomic DNA.
[0047] (2) Digest the total DNA with restriction endonuclease Sau3AI to form GATC cohesive ends. By controlling the enzyme dosage and reaction time, the total DNase is cut into 2-6kb fragments and recovered. These fragments were ligated with the pET28a plasmid digested by BamH I (recognition sequence GGATCC, cohesive end GATC), and the ligated product was transformed into competent cells E.coliBL21 ((DE3) After culturing at 37°C for 12-16 hours on a plain LB solid plate, pick a single colony for the next step of activity screening.
[0048] (3) Pick a single colony and inoculate i...
Embodiment 2
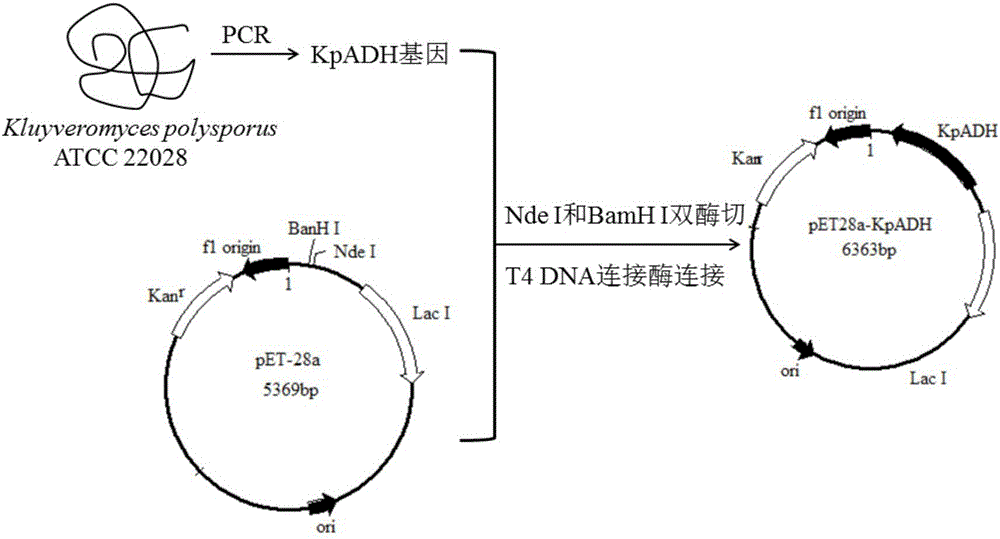
[0054] Example 2 Construction and cultivation of recombinant Escherichia coli BL21(DE3) / pET28a-kpadh
[0055] The gene kpadh recovered in Example 1 and the plasmid pET28a were double digested with restriction enzymes NdeI and BamH I in a water bath at 37°C overnight, purified by agarose gel electrophoresis the next day, and the target was recovered using an agarose recovery kit fragment. 4°C, use T 4 DNA ligase overnight ligase-cut gene kpadh and plasmid pET28a to obtain the recombinant expression vector pET28a-kpadh ( figure 2 ). The constructed recombinant expression vector pET28a-kpadh was thermally transferred into Escherichia coli BL21 (DE3) competent, coated with Kan-resistant LB solid plate, and colony PCR verification was carried out after overnight culture. The positive clone was the recombinant Escherichia coli BL21 ( DE3) / pET28a-kpadh. Pick positive clones and culture them overnight in LB medium, then transfer them into fresh LB culture at 2% transfer amount th...
Embodiment 3
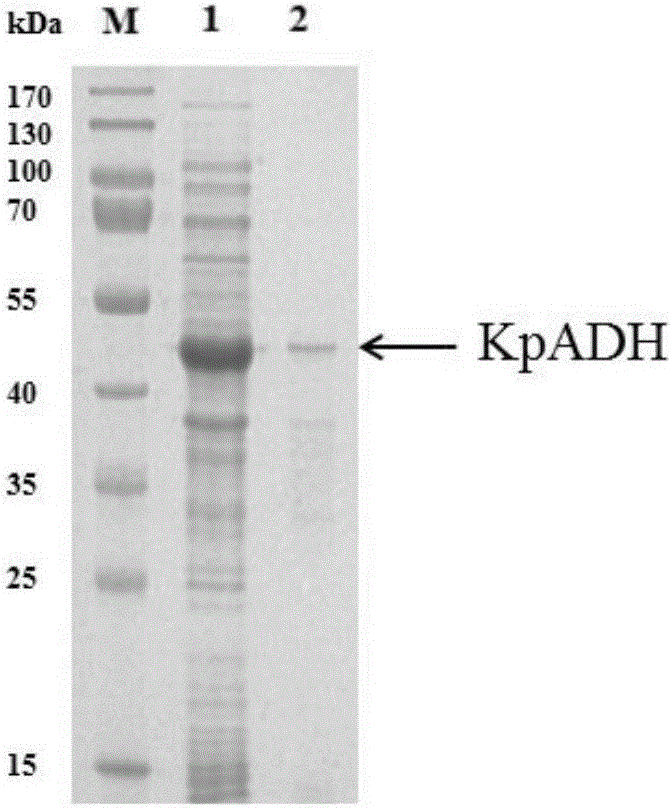
[0056] The separation and purification of embodiment 3 alcohol dehydrogenase
[0057] Suspension-cultured recombinant cells were placed in liquid A (20mmol·L -1 Sodium phosphate, 500mmol·L -1 NaCl, 20mmol·L -1 imidazole, pH 7.4), the crude enzyme solution was obtained after sonication and centrifugation. The column used for purification is an affinity column HisTrap FFcrude (nickel column), which is accomplished by using the histidine tag on the recombinant protein for affinity binding. First, use solution A to equilibrate the nickel column, load the crude enzyme solution, continue to use solution A to elute the breakthrough peak, and after equilibrium, use solution B (20mmol L -1 Sodium phosphate, 500mmol·L -1 NaCl, 1000mmol·L -1 imidazole, pH 7.4) for gradient elution to elute the recombinant protein bound to the nickel column to obtain recombinant alcohol dehydrogenase. Enzyme activity assay (CPMK as substrate, NADPH as coenzyme) and SDS-PAGE analysis were carried out...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com