Chemical synthesis method of (1R, 2S)-2-aryl cyclopropylamine derivative
An arylcyclopropylamine, a technology for chemical synthesis, applied in chemical instruments and methods, purification/separation of amino compounds, organic chemistry, etc., and can solve problems such as low stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
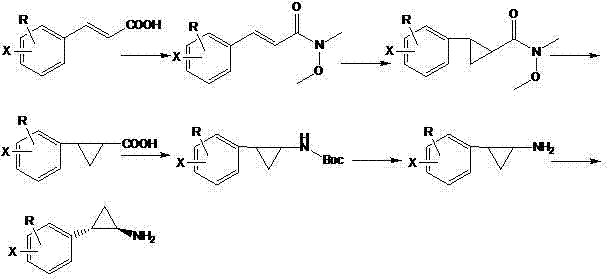
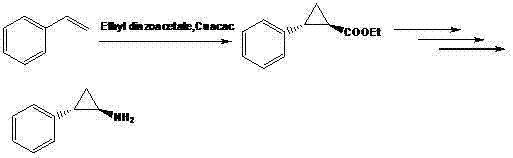
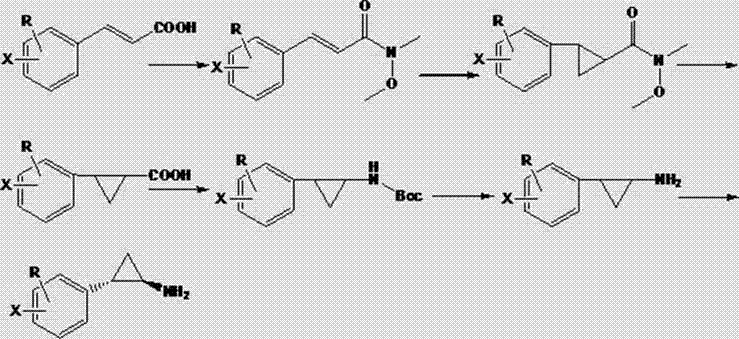
[0019] Synthesis of 2-phenyl-cyclopropylcarboxylic acid: Add 10.0 g NaH and 250 ml dimethyl sulfoxide into a three-necked flask under nitrogen protection, and add N-methoxy-N methyl-3-phenylpropene in batches amide 27.4 g, stirred and dissolved for 30 min, then added dropwise 42.0 g trimethylsulfoxide iodide, and reacted at room temperature. After adding water to quench and extract with methyl tert-butyl ether, the organic phase was washed with water three times, dried and concentrated to obtain 25.0 g of light yellow liquid with a yield of 85.0%. Transfer the yellow product to a single-necked bottle, add 200 ml of methanol and 100 ml of water, stir to dissolve, then add 10.0 g of sodium hydroxide, and stir to react overnight. After the reaction was completed, water was added to adjust the pH to 3-4, extracted with dichloromethane, and concentrated to obtain 17.8 g of white 2-phenyl-cyclopropyl formic acid solid with a yield of 90.1%.
[0020] Synthesis of N-Boc-2-phenylcyclo...
Embodiment 2
[0024] Synthesis of 2-(4'-methoxyphenyl)-cyclopropanecarboxylic acid: Add 10.0 g NaH and 300 ml dimethyl sulfoxide into a three-necked flask under nitrogen protection, and add N-methoxy-N 31.7 g of methyl-3-(4'-methoxyphenyl)acrylamide was stirred and dissolved for 30 min, then 42.0 g of trimethylsulfoxide iodide was added dropwise, and reacted at room temperature. It was quenched by adding water, extracted with methyl tert-butyl ether, washed with water three times, dried and concentrated to obtain 30.0 g of yellow liquid with a yield of 89.0%. Transfer the yellow product to a single-necked bottle, add 250 ml of methanol and 125 ml of water, stir to dissolve, then add 10.0 g of sodium hydroxide, and stir to react overnight. After the reaction was completed, water was added to adjust the pH to 3-4, extracted with dichloromethane, and concentrated to obtain 22.3 g of light yellow 2-(4'-methoxyphenyl)cyclopropanecarboxylic acid solid, with a yield of 91.0%.
[0025] Synthesis o...
Embodiment 3
[0029]Synthesis of 2-(4'-bromophenyl)-cyclopropanecarboxylic acid: Add 10.0 g NaH and 350 ml dimethyl sulfoxide into a three-necked flask under nitrogen protection, and add N-methoxy-N methyl - 38.7 g of 3-(4'-bromophenyl) acrylamide, stirred and dissolved for 30 min, then added dropwise 42.0 g of trimethylsulfoxide iodide, and reacted at room temperature. It was quenched by adding water, extracted with methyl tert-butyl ether, washed three times with water, dried and concentrated to obtain 32.3 g of yellow liquid with a yield of 79.3%. Transfer the yellow product to a single-necked bottle, add 300 ml of methanol and 150 ml of water, stir to dissolve, then add 10.0 g of sodium hydroxide, and stir to react overnight. After the reaction was completed, adjust the pH to 3-4, extract with dichloromethane, and concentrate to obtain 24.3 g of light yellow 2-(4'-bromophenyl)cyclopropyl formic acid solid, with a yield of 88.6%.
[0030] Synthesis of N-Boc-2-(4'-bromophenyl)cyclopropyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com