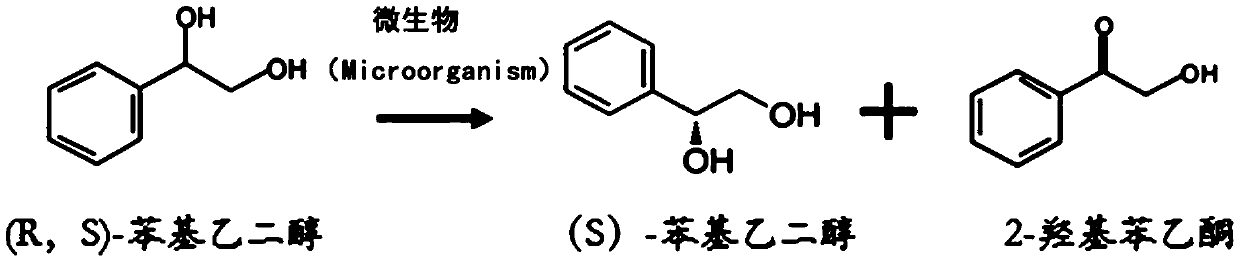
A method for preparing (s)-phenylethylene glycol by asymmetric separation of microorganisms
A phenylethylene glycol, asymmetric technology, applied in the field of preparation of (S)-phenylethylene glycol by asymmetric separation of microorganisms, can solve the problems of low reaction yield, low substrate solubility, toxic effects, etc. Achieve the effect of improving enantiomeric purity and yield, increasing substrate concentration, and inhibiting product toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Bacterial Strain Cultivation
[0046] Prepare seed medium and fermentation medium according to the following formulas:
[0047] SC0312 seed medium: Take 0.15g of yeast extract, 0.25g of NaCl and 0.5g of tryptone, add it into distilled water to make 50mL of seed medium, and sterilize at 121°C for 20min.
[0048] SC0312 fermentation medium: Take 0.3g of yeast extract, 0.5g of NaCl and 1g of tryptone, add it into distilled water to make 100mL of fermentation medium, and sterilize at 121°C for 20min.
[0049] Take 1‰ (v / v) (50μL) of Kouterella gibsonii and add it to 50mL seed medium, shake and cultivate at 37°C and 180rpm for 10-12h to obtain the seed culture solution; then add 1‰ (v / v ) (100 μL) inoculum size, the seed culture solution was added to 100 mL of fermentation medium, cultured at 37° C. and 180 rpm for 12 to 16 hours, and the fermentation solution was harvested.
[0050] The fermentation broth was centrifuged at 4° C. and 7900 rpm for 10 min to colle...
Embodiment 2
[0051] The influence of embodiment 2 organic solvents on biocatalytic reaction
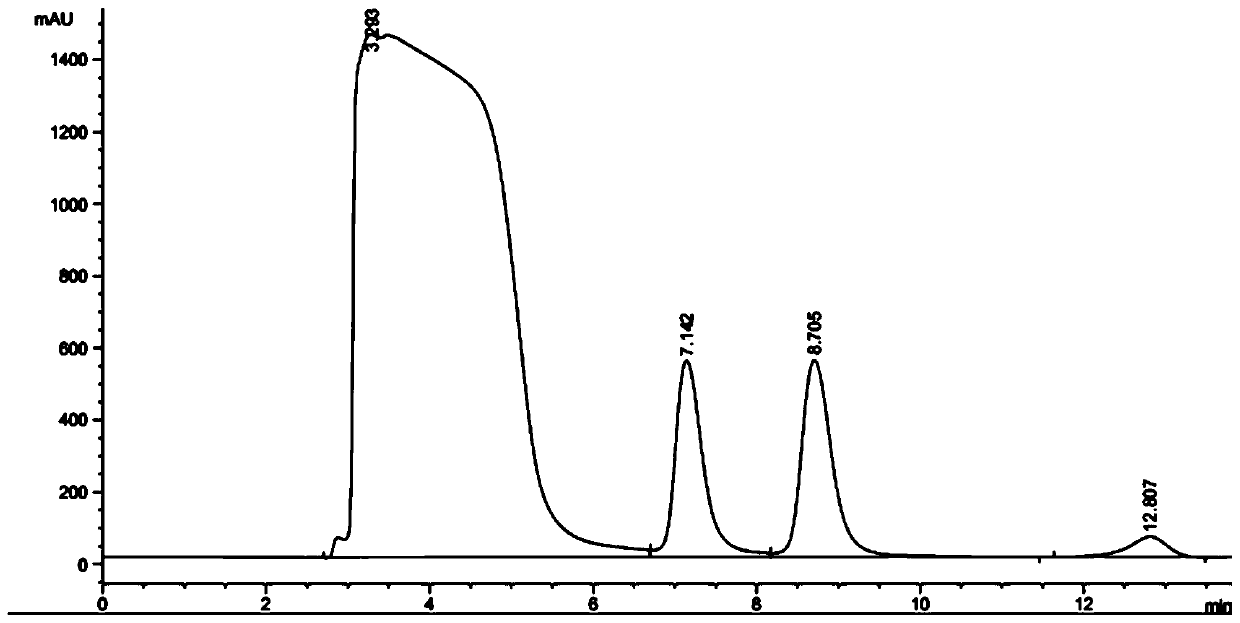
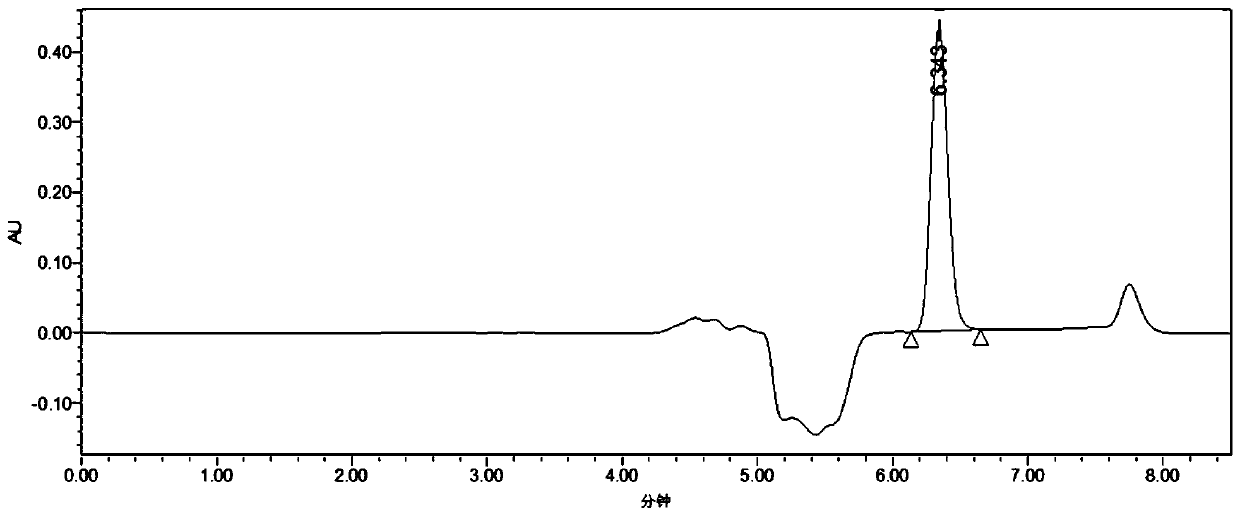
[0052] Add 2mL of 100mM, pH 6.5 phosphate buffer and 2mL of organic solvents (tert-amyl alcohol, n-hexanol, 2-methyltetrahydrofuran, dibutyl phthalate, dimethyl carbonate, butyl acetate, etc. ester, isooctane, n-decane, dipentene) to form a biphasic system with a volume ratio of 1:1, and then add 11.05 mg of racemic (R, S)-phenylethylene glycol (that is, the final concentration 20mM) and 0.10g of Gitkut bacteria (that is, the final concentration is 25mg / mL), reacted at 35°C and 180rpm for 6h, and after the reaction was completed, the product (S)-phenylben The enantiomeric excess of diol is respectively 0.05%, 0.03%, 0.04%, 99.99%, 0.02%, 0.54%, 0.71%, 99.99%, 0.03% (the product liquid phase chromatogram is as follows figure 1 and 2 shown).
Embodiment 3
[0053] The influence of embodiment 3 reaction initial pH on biocatalytic reaction
[0054] Add 2mL of 100mM to a 25mL serum bottle, then add phosphate buffer solution of different pH (pH is 4.5, 5, 5.5, 6.5, 7.5, 8.5) and 2mL of dibutyl phthalate to form a volume ratio of 1 : 1 biphasic system, then add 11.05mg of racemic (R, S)-phenylethylene glycol (final concentration is 20mM) and 0.12g Gitkutella (final concentration is 30mg / mL), Reacted at 35°C and 180rpm for 6h. After the reaction was over, the enantiomeric excess of the product (S)-phenylethylene glycol detected by HPLC was 98.67%, 98.38%, 99.99%, 99.99%, respectively. 99.99%, 99.99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com