Method for synthesizing propargylamine derivative with different substituent groups at alkyne terminal
A technology of propargylamine and substituents, which is applied in the field of mutual conversion between different propargylamines, can solve the problems of unsuitable modification of end group of internal alkyne-type propargylamine, and achieves easy commercial purchase or preparation, high yield and moderate price Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
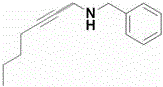
[0030] The preparation of N-benzyl-2-heptynylamine, structural formula is as follows:
[0031]
[0032] Under nitrogen protection, add raw material 1-hexyne (1.5mmol), N-benzyl-2-butynylamine (0.3mmol) and catalyst Lu[N(SiMe 3 ) 2 ] 3 (1mol%), xylene (2ml), reacted at 150°C for 48h, and the isolated yield of the product was 70%.
[0033] 1 HNMR (CDCl 3 ,400MHz,ppm):δ7.35-7.30(m,4H),7.27-7.25(m,1H),3.86(s,2H),3.40(t, J =2.12Hz,2H),2.23-2.19(m,2H),1.62(br,1H),1.52-1.40(m,4H),0.92(t, J =7.21Hz, 3H).
Embodiment 2
[0035] The preparation of N-benzyl-5-phenyl-2-pentynylamine, structural formula is as follows:
[0036]
[0037] Under nitrogen protection, add raw material phenylethylacetylene (1.5mmol), N-benzyl-2-butynylamine (0.3mmol) and catalyst Lu[N(SiMe 3 ) 2 ] 3 (10mol%), toluene (2ml), reacted at 130°C for 12h, and the isolated yield of the product was 95%.
[0038] 1 HNMR (CDCl 3 ,400MHz,ppm):δ7.31-7.20(m,10H),3.80(s,2H),3.38(t, J =2.14Hz,2H),2.83(t, J =7.51Hz, 2H), 2.52-2.49(m, 2H), 1.47(br, 1H).
Embodiment 3
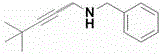
[0040] The preparation of N-benzyl-4,4-dimethyl-2-pentynylamine, the structural formula is as follows:
[0041]
[0042] Under the protection of nitrogen, the raw material tert-butylacetylene (1.5mmol), N-benzyl-2-butynylamine (0.3mmol) and catalyst Sc[N(SiMe 3 ) 2 ] 3 (10mol%), toluene (2ml), reacted at 130°C for 12h, and the isolated yield of the product was 80%.
[0043] 1 HNMR (400MHz, CDCl 3 )δ7.34-7.32 (m, 4H), 7.27-7.24 (m, 1H), 3.86 (s, 2H), 3.39 (s, 2H), 1.65 (br, 1H), 1.24 (s, 9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com