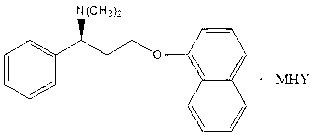
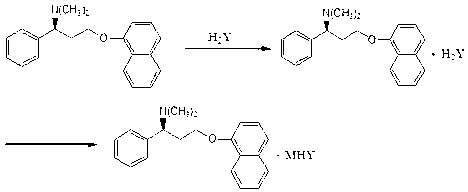
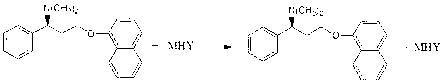Naphthyloxy benzedrine derivatives and preparation method thereof
A technology of naphthoxyamphetamine and derivatives, which is applied in the field of compound preparation and achieves the effects of high bioavailability, high purity and fast absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037] In a 100ml reaction bottle, add 305.42mg of naphthyloxyamphetamine, dissolve it with 50ml of anhydrous methanol, stir, add 98mg of sulfuric acid, after the reaction is completed, recover and concentrate to obtain 399mg of naphthyloxyamphetamine sulfate, and then add naphthyloxyamphetamine acid salt Mix in acetone, add 54mg of sodium methoxide to react for 2 hours, concentrate under reduced pressure, add an appropriate amount of ether, precipitate the solid, filter, wash with ether, and dry to obtain 422mg of white solid naphthyloxyamphetamine sodium bisulfate double salt, yield 92%.
Embodiment 2
[0039]
[0040] In a 100ml reaction bottle, add 305.42mg of naphthyloxyamphetamine, dissolve it with 50ml of absolute ethanol, stir, add 98mg of sulfuric acid, after the reaction is completed, recover and concentrate to obtain 399mg of naphthyloxyamphetamine sulfate, and then add naphthyloxyamphetamine acid salt Mix in DMF, add 84 mg of potassium ethylate to react for 2 hours, concentrate under reduced pressure, add appropriate amount of petroleum ether, precipitate solid, filter, wash with petroleum ether, and dry to obtain 386 mg of white solid naphthyloxyamphetamine potassium hydrogensulfate double salt, Yield 80%.
Embodiment 3
[0042]
[0043] In a 100ml reaction bottle, add 305.42mg of naphthyloxyamphetamine, dissolve with 50ml of anhydrous DMF, stir, add 98mg of sulfuric acid, after the reaction is completed, recover and concentrate to obtain 399mg of naphthyloxyamphetamine sulfate, and then add naphthyloxyamphetamine acid salt Mix in ethanol, add 92 mg of ammonium propionate to react for 3 hours, concentrate under reduced pressure, add an appropriate amount of n-hexane, precipitate the solid, filter, wash with n-hexane, and dry to obtain 319 mg of white solid naphthyloxyamphetamine ammonium bisulfate double salt , yield 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com