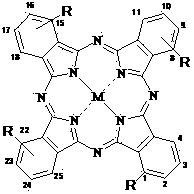
Phthalocyanine metal complex containing piperazine ethyoxyl modification group and preparing method thereof
A technology of metal complexes and substituent groups, applied in the field of phthalocyanine metal complexes and its preparation, can solve the problems of high skin phototoxicity, poor bioselectivity, lack of amphiphilicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Synthesis and Physicochemical Properties of 1,8(11), 15(18), 22(25)-tetrakis[2-(N-tert-butoxycarbonylpiperazinyl)ethoxy]zinc phthalocyanine
[0051]
[0052] Formula 1)
[0053] This compound can also be called four-a-[2-(N-tert-butoxycarbonylpiperazinyl)ethoxy]zinc phthalocyanine, and its structure is shown in formula (1), wherein:
[0054] .
[0055] (1) Protect the terminal amino group of N-hydroxyethylpiperazine by BOC (tert-butoxycarbonyl): in 20~60ml (preferably 30 ml) CH 2 Cl 2 Add 20mmol N-hydroxyethylpiperazine and 20~40mmol (preferably 30 mmol) triethylamine, then slowly add 20~50mmol (preferably 40 mmol) di-tert-butyl dicarbonate dropwise, and stir at 0°C for 0.5~2 hours (Preferably 1 hour), then stir the reaction at 30°C, monitor by thin-layer chromatography, and stop the reaction when the N-hydroxyethylpiperazine is substantially consumed. Removal of CH by rotary evaporation 2Cl 2 and triethylamine, the obtained pale yellow oily liquid was washed...
Embodiment 2
[0061] Synthesis and Physicochemical Properties of 1,8(11), 15(18), 22(25)-Tetrakis(2-piperazinylethoxy)zinc Phthalocyanine
[0062] This compound can also be called four-a-(2-piperazinylethoxy)zinc phthalocyanine, and its structure is shown in formula (1), wherein:
[0063] .
[0064] Under nitrogen protection conditions, 0.25mmol tetra-a-[2-(N-tert-butoxycarbonylpiperazinyl)ethoxy]zinc phthalocyanine obtained in Example 1 was added to 5 ml tetrabutylammonium fluoride (TBAF) in tetrahydrofuran solution, the concentration of tetrabutylammonium fluoride is 0.5~2M (preferably 1M), stirred and refluxed for 5~10 hours, and the reaction end point is monitored by thin layer chromatography. The solvent was removed by rotary evaporation, washed with water, and freeze-dried under reduced pressure. Then use THF as the mobile phase to purify through a gel column, collect dark green components, and dry to obtain 122 mg of product with a yield of 45%. The maximum absorption peak of th...
Embodiment 3
[0067] Synthesis and Physicochemical Properties of 1-[2-(N-tert-butoxycarbonylpiperazinyl)ethoxy]zinc Phthalocyanine
[0068]
[0069] Formula (2)
[0070] The structure of the compound is shown in formula (2), wherein:
[0071] .
[0072] (1) Protect the terminal amino group of N-hydroxyethylpiperazine by BOC (tert-butoxycarbonyl): in 20~60ml (preferably 30 ml) CH 2 Cl 2 Add 20mmol N-hydroxyethylpiperazine and 20~40mmol (preferably 30 mmol) triethylamine, then slowly add 20~50mmol (preferably 40 mmol) di-tert-butyl dicarbonate dropwise, and stir at 0°C for 0.5~2 hours (Preferably 1 hour), then stir the reaction at 30°C, monitor by thin-layer chromatography, and stop the reaction when the N-hydroxyethylpiperazine is substantially consumed. Removal of CH by rotary evaporation 2 Cl 2 and triethylamine, the obtained pale yellow oily liquid was washed with CH 2 Cl 2 Dissolve, wash three times with water, collect the organic phase, anhydrous MgSO 4 After drying, the s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com