Synthetic method of tomoxetine
A technology of atomoxetine and compounds, which is applied in the field of synthesis of drugs for the treatment of attention deficit hyperactivity disorder (ADHD), can solve problems such as increased production costs, difficulty in control, and unsatisfactory yields, and achieves the reduction of by-products, The effect of mild reaction conditions and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
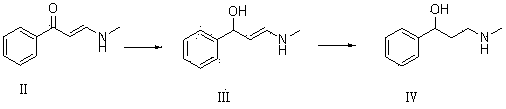
[0042] 10g of compound I and 20g of ethanol solution containing 33% methylamine were dissolved in 60mL of ethanol for reaction. TLC determined that the reaction was complete, adding an appropriate amount of water, extracting with dichloromethane, drying over anhydrous magnesium sulfate, and concentrating to obtain 10.52g of oily compound II. Yield 85%.
[0043] 10g of compound II and 9.4g of sodium borohydride were dissolved in 50mL of tetrahydrofuran for reaction. TLC determined that the reaction was complete. Water was added and extracted with ethyl acetate. The organic phases were combined, dried and concentrated to obtain 7.94g of colorless oily compound III with a yield of 78.5%. .
[0044] Dissolve 7g of compound III and 0.7g of 10% Pd / C in 35mL of ethanol, pass through hydrogen to react, TLC determines that the reaction is complete, filter off the catalyst, concentrate to obtain an oil, and recrystallize with petroleum ether to obtain 6.25g of compound Ⅳ, the yield 88....
Embodiment 2
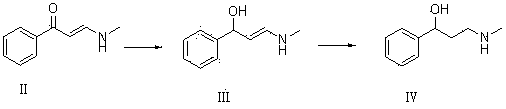
[0047] Dissolve 10g of compound I and 22g of ethanol solution containing 33% methylamine in 80mL of isopropanol for reaction. TLC determines that the reaction is complete. Add appropriate amount of water, extract with ethyl acetate, dry with anhydrous calcium chloride, and concentrate to obtain 10.42g of oil Compound II, yield 84.2%.
[0048] 9.5 g of compound II and 9.5 g of potassium borohydride were dissolved in 50 mL of ethanol for reaction. TLC determined that the reaction was complete, adding water, extracting with ethyl acetate, combining the organic phases, drying and concentrating to obtain 7.5 g of colorless oily compound III with a yield of 78 %.
[0049] 7g of compound III and 0.5g of Raney Ni were dissolved in 40mL of ethanol and reacted with hydrogen gas. TLC determined that the reaction was complete. The catalyst was filtered off, concentrated to obtain an oil, and recrystallized with petroleum ether to obtain 6.2g of compound IV with a yield of 87.4%.
[0050]...
Embodiment 3
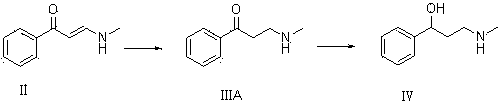
[0052]8 g of compound I and 23 g of ethanol solution containing 33% methylamine were dissolved in 50 mL of methanol for reaction. TLC determined that the reaction was complete, adding an appropriate amount of water, extracting with dichloromethane, drying over anhydrous magnesium sulfate, and concentrating to obtain 8.32 g of oily compound II. Yield 84%.
[0053] 7.5g of compound II and 8.8g of lithium aluminum hydride were dissolved in 60mL of methanol and reacted. TLC determined that the reaction was complete. Water was added and extracted with ethyl acetate. The organic phases were combined, dried and concentrated to obtain 5.9g of colorless oily compound III with a yield of 77.7% %.
[0054] 5g of compound III and 0.4g of 8% Pd(OH)2 / C were dissolved in 30mL of tetrahydrofuran and reacted. TLC determined that the reaction was complete. The catalyst was filtered off, concentrated to obtain an oil, and recrystallized with petroleum ether to obtain 4.43g of compound IV. The r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com