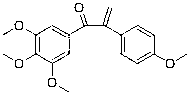
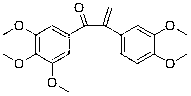
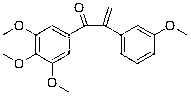
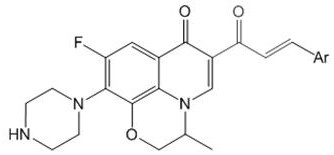
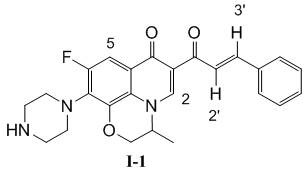
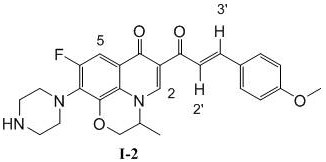
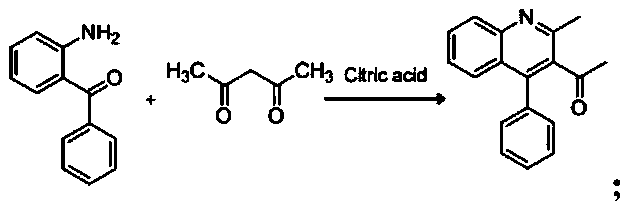
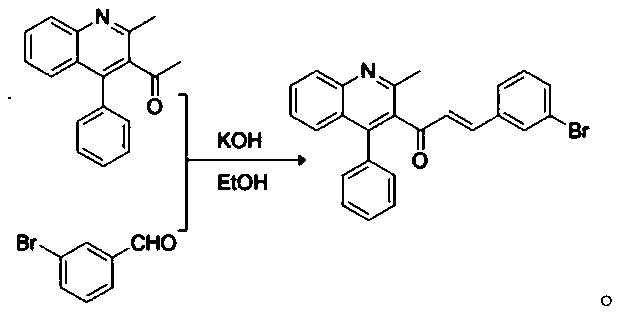
Patents
Literature
85 results about "Acrylophenone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
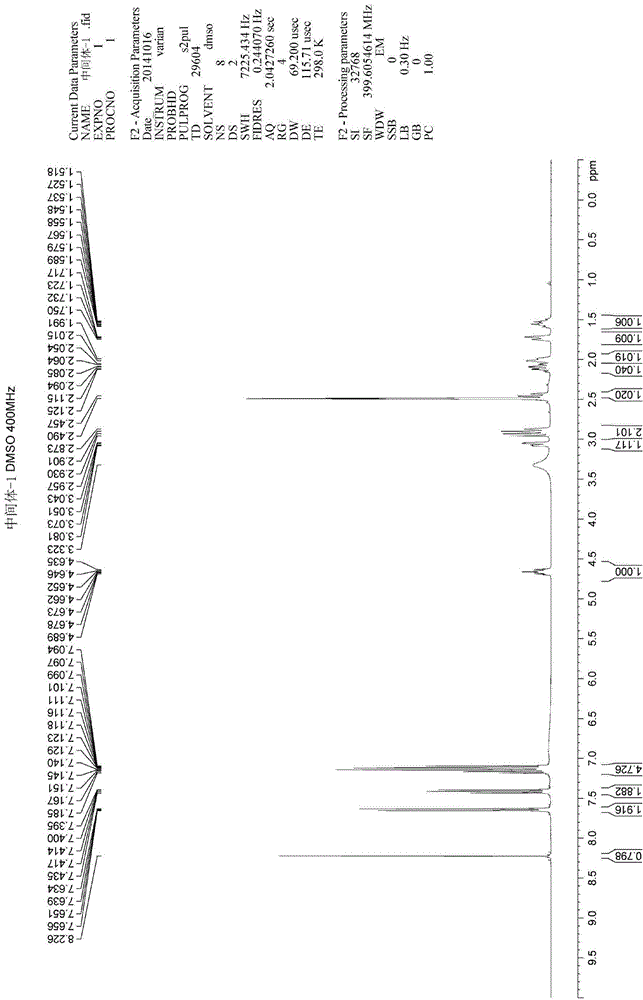
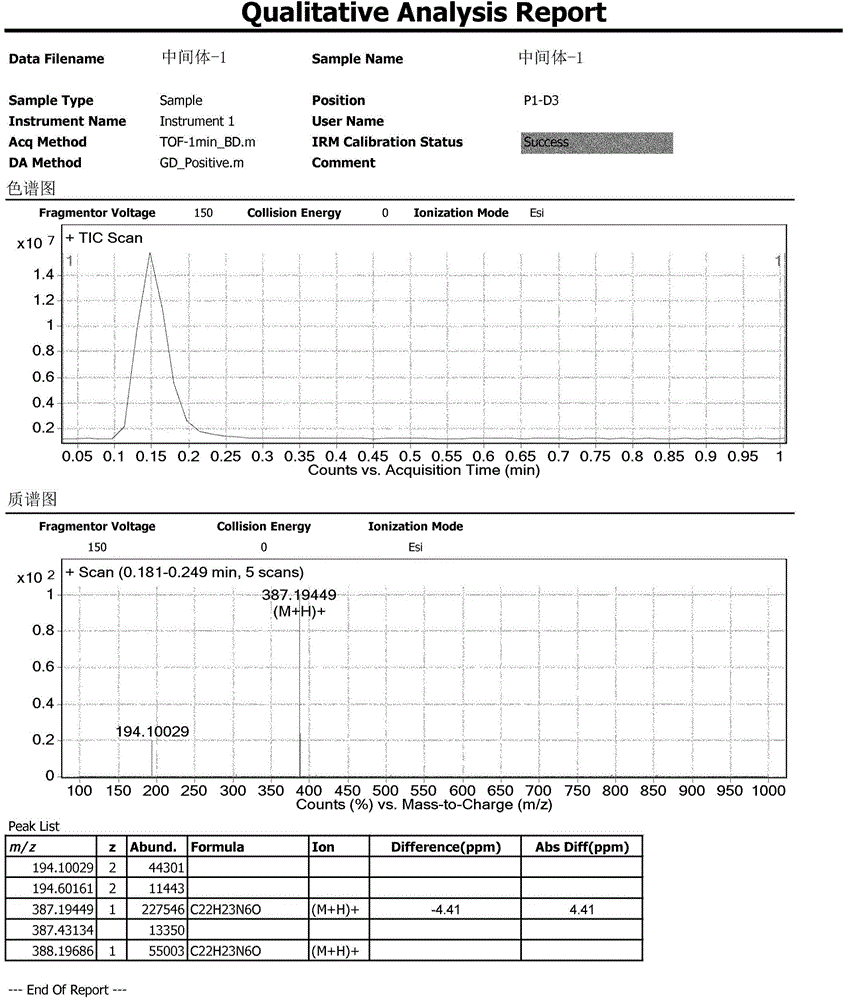
Acrylophenone is an organic compound with the formula C₉H₈O. It is prepared using acetophenone, formaldehyde, and an amine hydrochloride in a Mannich reaction. It can be polymerized to poly(phenylvinyl ketone) via radical or anionic mechanisms. It is sometimes used as a comonomer in the manufacturing of certain resins.
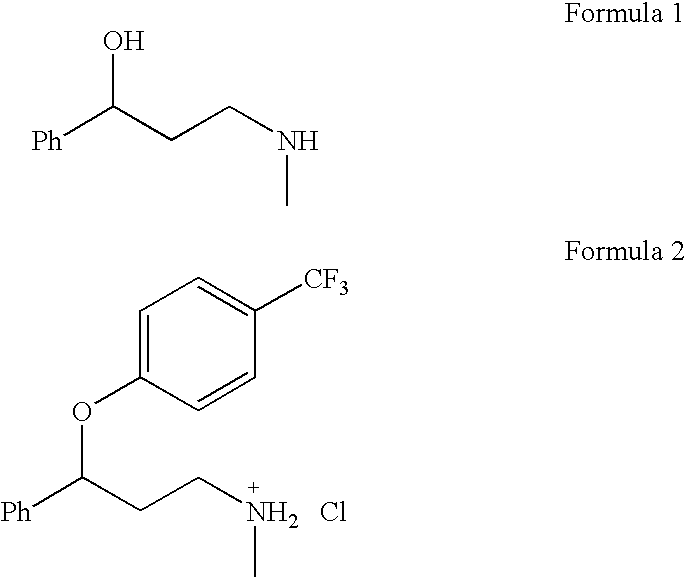
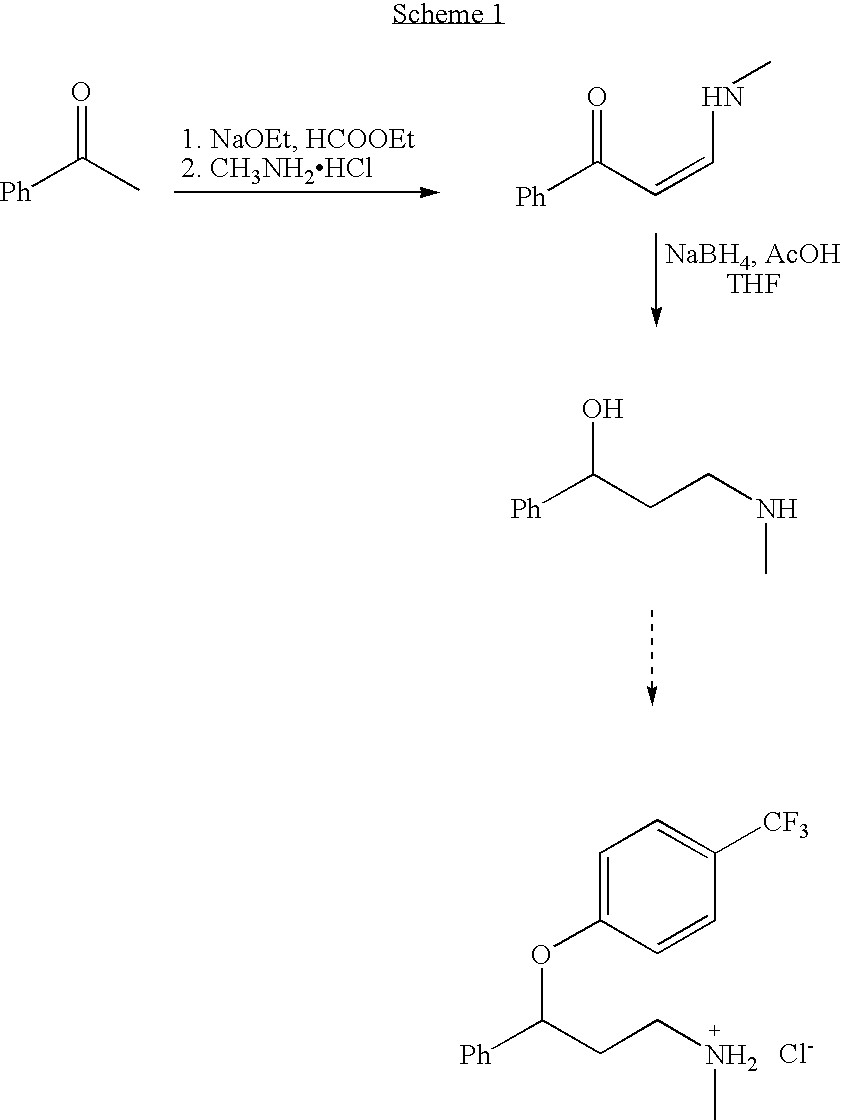
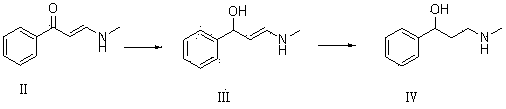
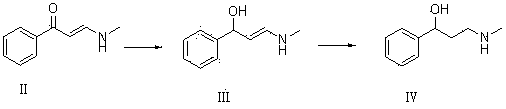
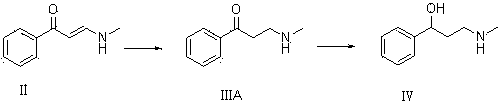
Synthesis of 3-aminomethyl-1-propanol, a fluoxetine precursor
The present invention concerns a method of synthesizing fluoxetine hydrochloride. The method includes the synthesis of 3-methylamino-1-phenyl-1-propanol by reduction of 1-phenyl-3-methylamino-2-propen-1-one with sodium borohydride and acetic acid.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
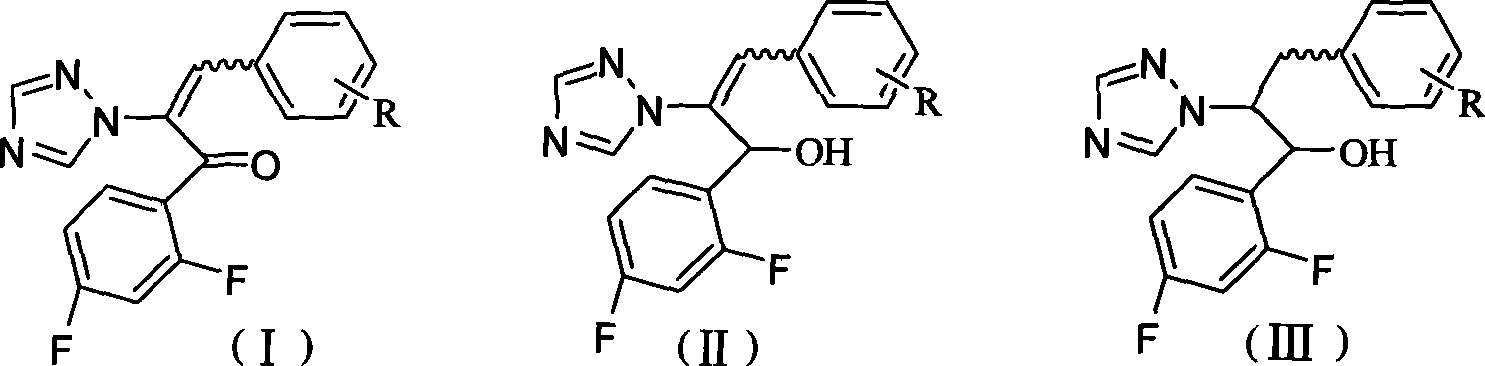
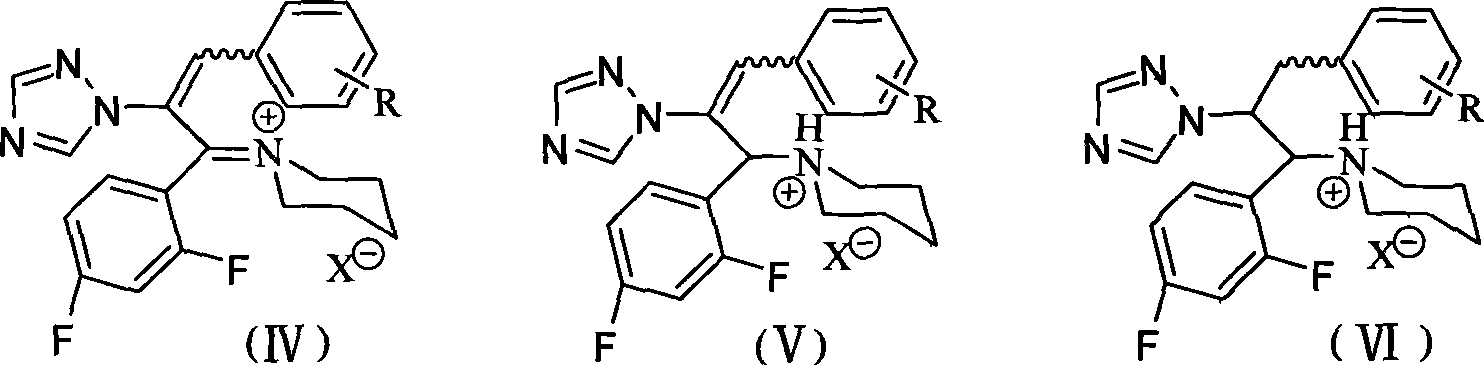
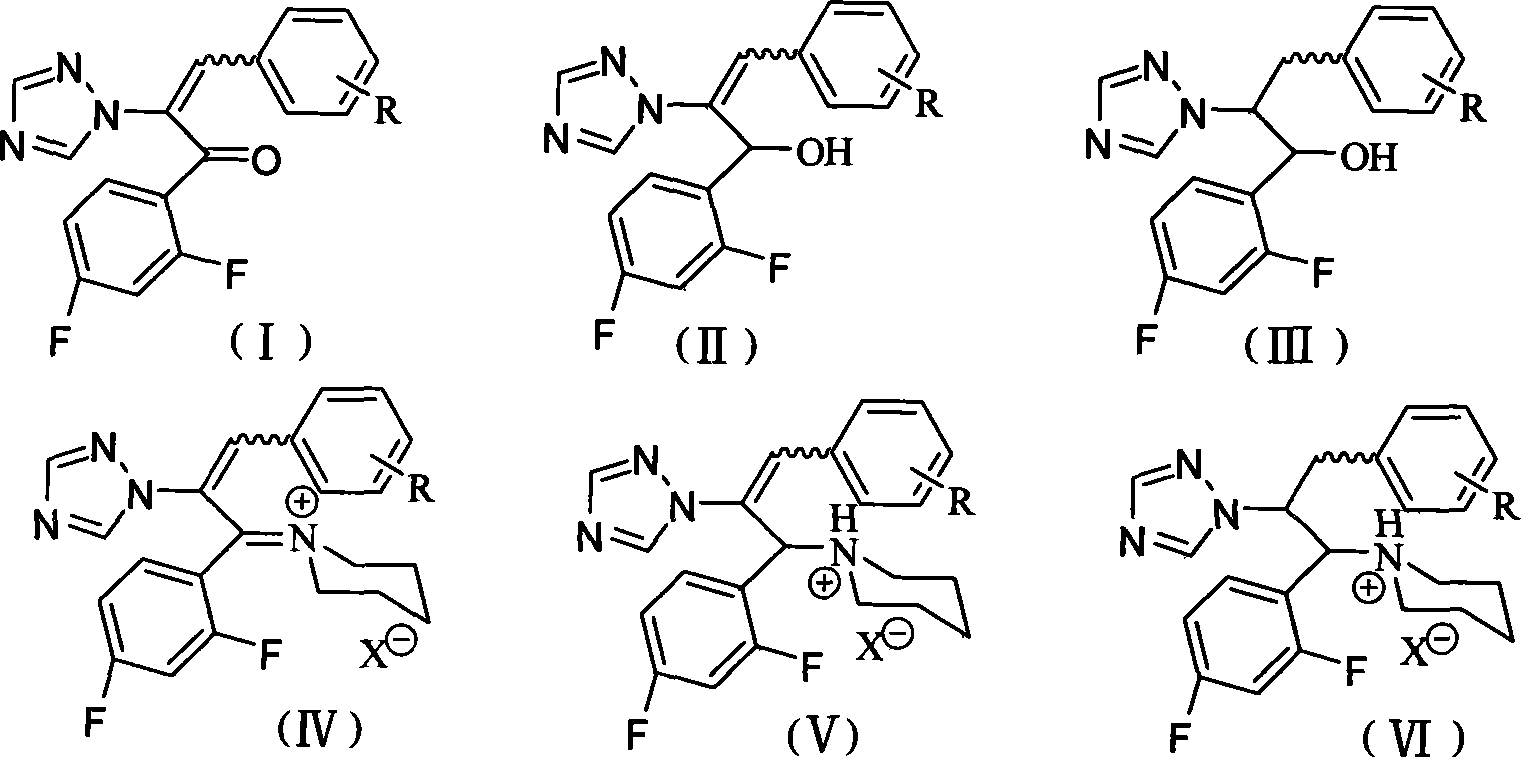
Triazoles compounds with antimicrobial activity and preparation method and pharmaceutical use thereof
InactiveCN101445488AThe synthesis method is simpleRaw materials are easy to getOrganic active ingredientsOrganic chemistryMedicinal chemistryPharmaceutical Plants
The invention discloses novel triazoles compounds, whose chemical names are 3-(substituted phenyl)-1-(2,4-difluorophenyl)-2-(1,2,4-triazol-1-yl)-2-propen-1-one, 1-[3-(substituted phenyl)-1-(2,4-difluorophenyl)-2-(1,2,4-triazol-1-yl)allyl]piperidinium, 3-(substituted phenyl)-1-(2,4-difluorophenyl)-2-(1,2,4-triazol-1-yl)-2-propen-1-ol, and 1-[3-(substituted phenyl)-1-(2,4-difluoropheny)-2-(1,2,4-triazol-1-yl)proyl]piperidinium, with antimicrobial activity; further discloses the preparation method and pharmaceutical use thereof.
Owner:SOUTHWEST UNIVERSITY
Fluorescent probe PMPA as well as preparation method and application of fluorescent probe PMPA
InactiveCN106800531AThe synthesis steps are simpleLow costOrganic chemistryFluorescence/phosphorescenceAlcoholFluorescence
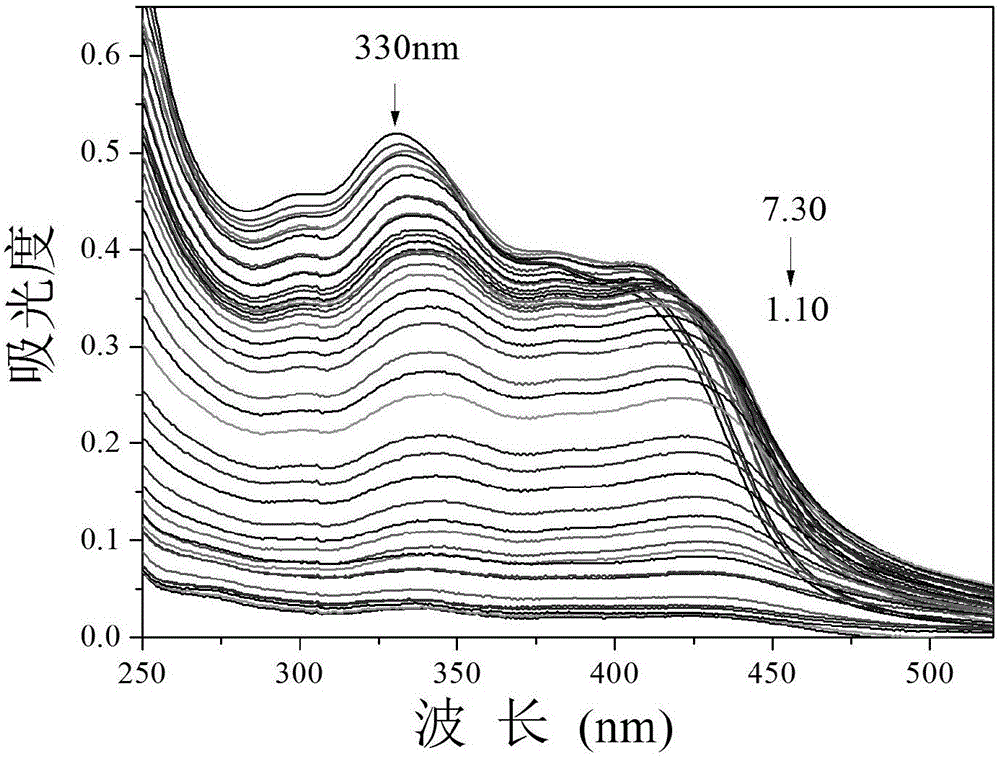
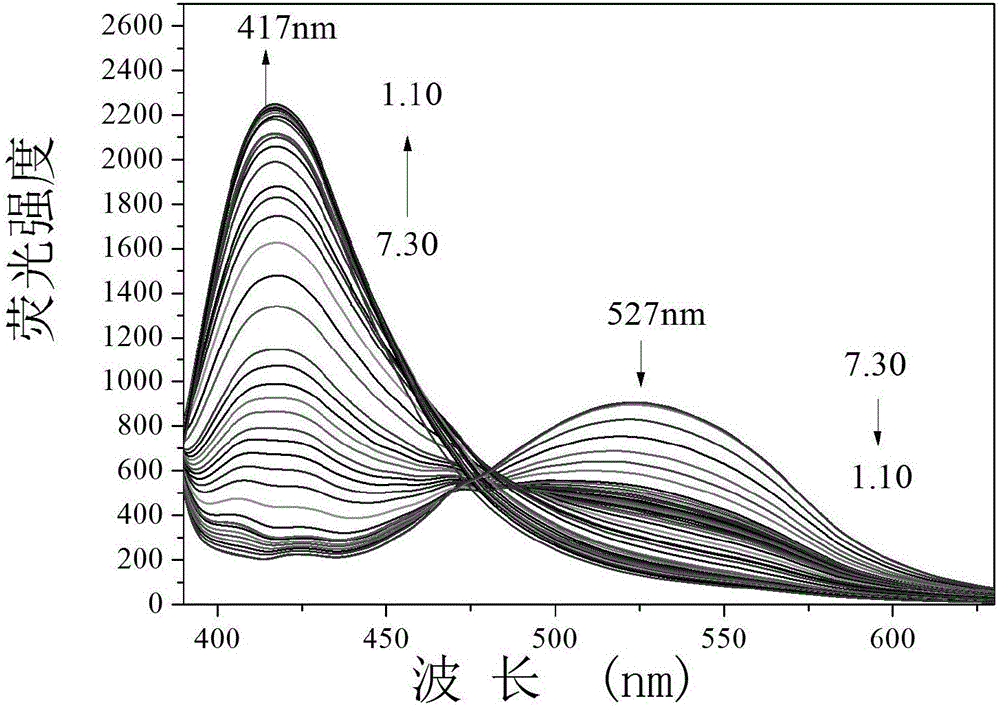
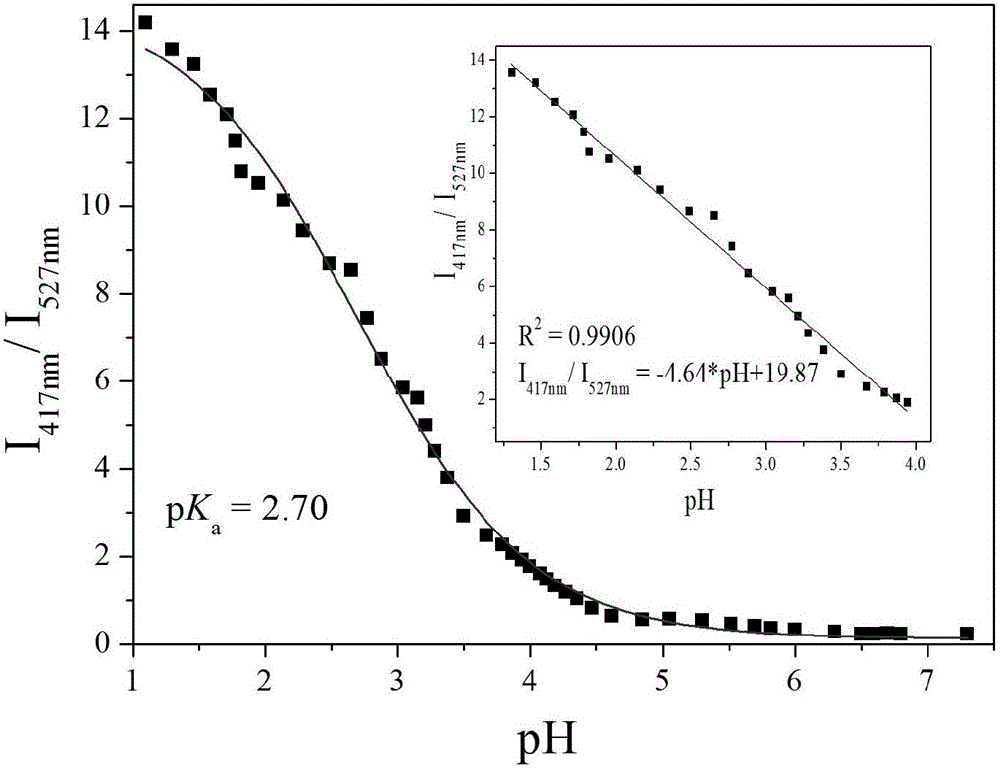
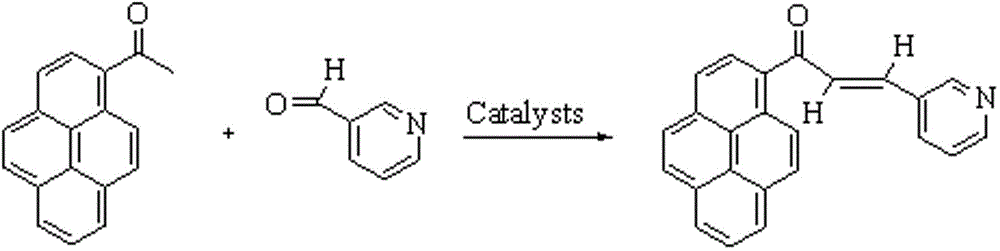
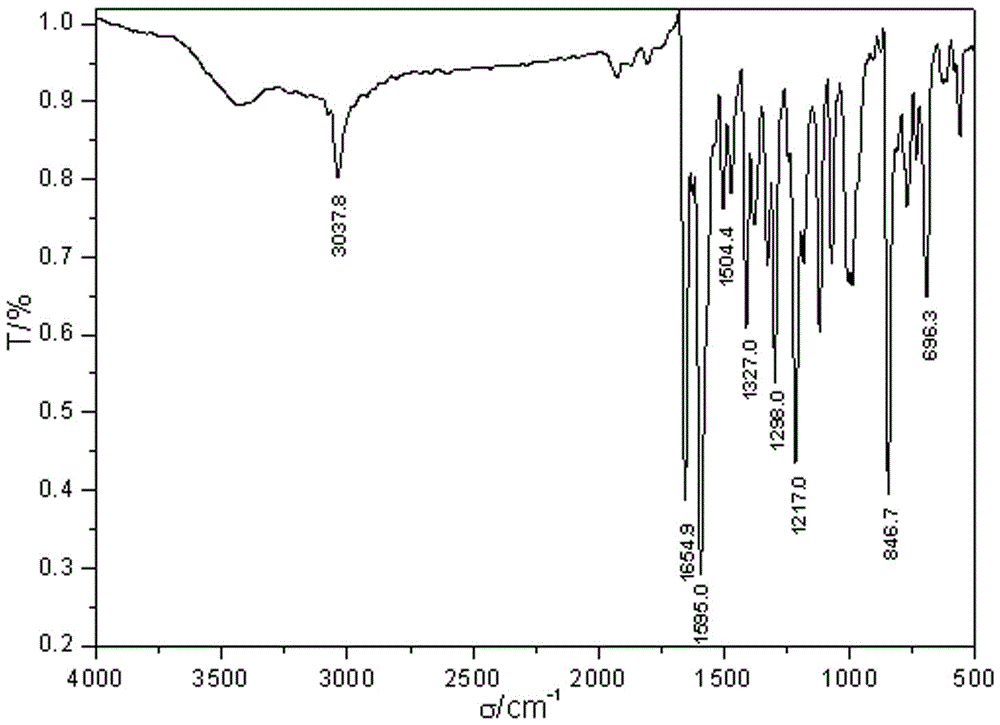
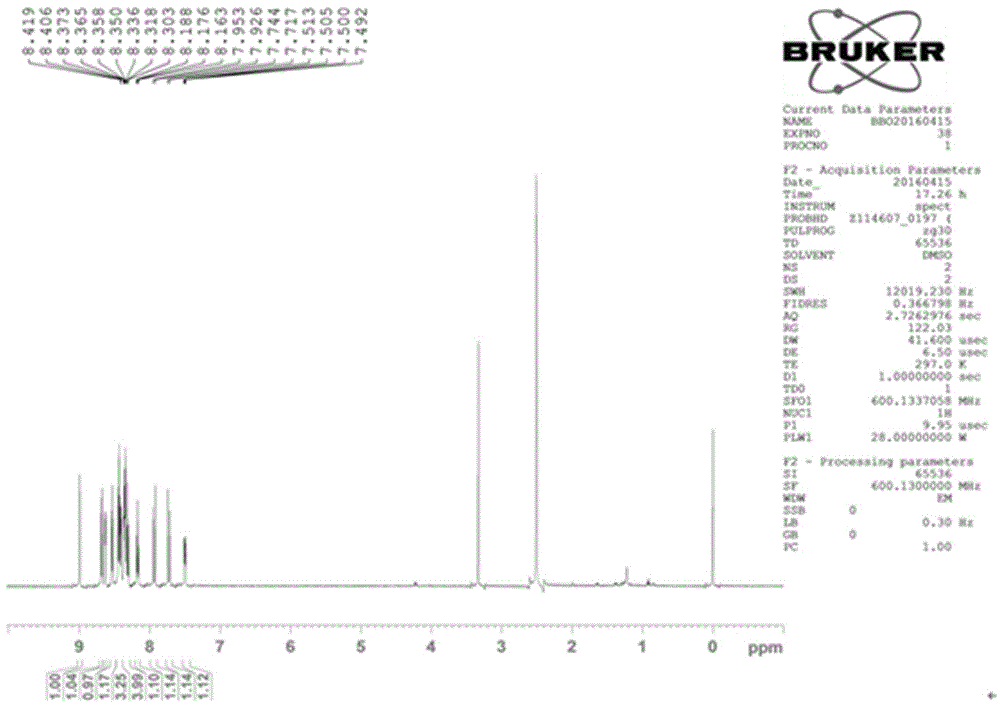
The invention provides a fluorescent probe PMPA as well as a preparation method and application of fluorescent probe PMPA. The fluorescent probe is 1-(pyrene-1-yl)-3-(6-methoxypyridine-3-yl) acrylketone. The preparation method comprises the steps of dissolving 1-acetylpyrene and 6-methoxy-pyridinedicarboxaldehyde into absolute ethyl alcohol in the molar ratio of 3:(3-5); adding a sodium hydroxide solution, further stirring at a room temperature for 32h and carrying out TLC tracking reaction; finally adding the absolute ethyl alcohol to generate a bright yellow flocculent; and carrying out standing, suction filtration and dying to obtain a yellow solid. Detection of the fluorescent probe on pH is rate type, detection on HSO3- is fluorescence quenching, and the fluorescent probe can be applied to detection of pH and HSO3- changes in a biological sample through combining a laser confocal microscopy.
Owner:SHANXI UNIV
Synthetic method of tomoxetine
ActiveCN103664658AStable in natureSimple stepsOrganic compound preparationAmino-hyroxy compound preparationCombinatorial chemistryKetone
The invention relates to a synthetic method of tomoxetine. The tomoxetine is generated by taking (E)-3-(N-methyl amino)-1-phenyl-2-propylene-1-ketone as the material through reduction and 2-benzyl halide etherification reaction. The synthetic method disclosed by the invention is simple in step, easy to operate, less in byproducts, better in yield, lower in cost and suitable for large-scale industrial production.
Owner:BEIJING WINSUNNY PHARMA CO LTD
Analysis method for related substances of Ibrutinib and Ibrutinib preparations
The invention belongs to the field of medicine synthesis, in particular to an analysis method for impurities and Ibrutinib preparations in the Ibrutinib (1-[(3R)-3-[4-amino-3-(4-phenoxy-phenyl)-1Hquinoline [3,4-d]pyrimidine-1-yl]-1-piperidyl]-2-propylene-1-ketone) preparation process.
Owner:BEIJING CREATRON INST OF PHARMA RES CO LTD +1
Gamma-alkenyl substituted butenolide or butenolactam compound and asymmetric synthesis method and ligand of gamma-alkenyl substituted butenolide or butenolactam compound
ActiveCN111960909AOrganic-compounds/hydrides/coordination-complexes catalystsMetallocenesCycloadditionInsertion reaction
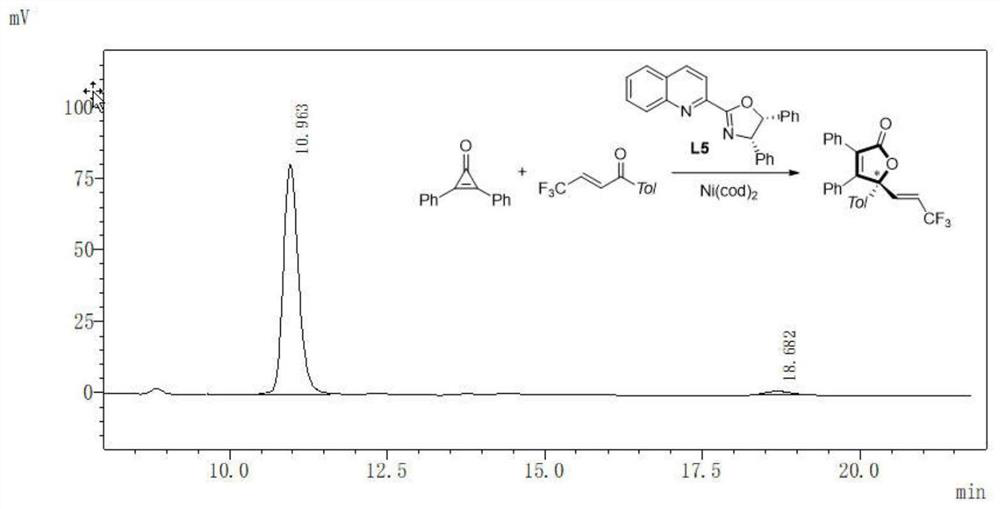
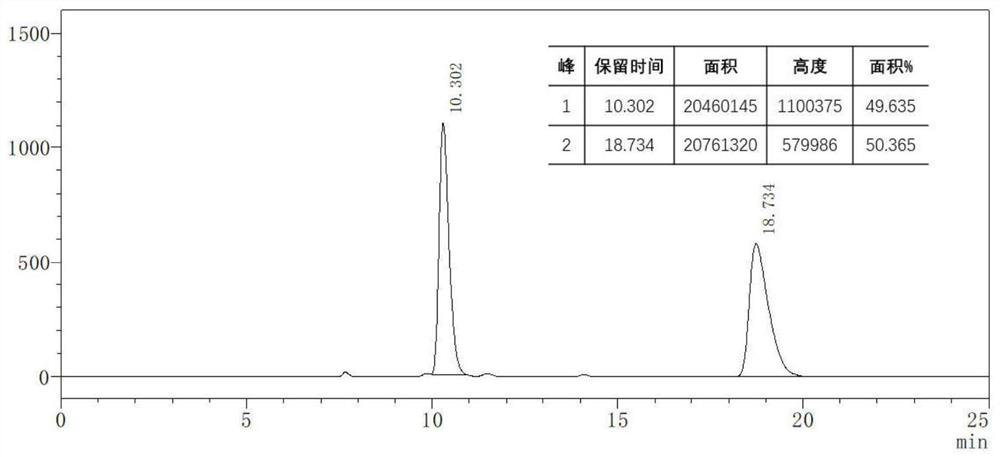
The invention discloses an asymmetric synthesis method of a gamma-alkenyl substituted butenolide or butenolactam compound. The method adopts cheap nickel to catalyze a [3+2] asymmetric cycloaddition reaction of a cyclopropenone compound and alpha, beta-unsaturated ketone or imine, the selective insertion reaction of intermolecular C=X after nickel-catalyzed C-C bond activation is realized for thefirst time, wherein X is equal to O or N, the gamma-alkenyl substituted butenolide or butenolactam compound is obtained in a high yield, high enantioselectivity and chirality controllable manner, thesynthesis method is novel, condition mildness, substrate with good applicability, simple and efficient reaction, cheap and readily available catalyst, according to the present invention, the synthesisprocess is simple, the atom economy is good, the synthesis product is easy to derivatize, the method can be widely used in completely-synthesized designed synthesis building blocks and new chiral drug derivatives, and the ligand compound is further provided, and can be used for the asymmetric synthesis of gamma-alkenyl substituted butenolide or butenolactam compounds.
Owner:HENAN NORMAL UNIV
Novel chalcone compound and application thereof
PendingCN107973708AImprove fatigue resistanceImprove spontaneous activityOrganic chemistryAntinoxious agentsAge related diseaseOxidation resistant
The invention provides a novel 2-propene-1-one substituted chalcone compound with an anti-aging effect. The novel chalcone compound can serve as a bulk drug for preparing related drugs with anti-oxidation and anti-aging functions of various dosage forms, and has the medical application of treating aging-related diseases.
Owner:王艳艳
Novel pyrenyl chalcone derivative and synthesis method thereof
InactiveCN106699644AMacromolecular polarizabilityOrganic chemistryLuminescent compositionsAlcoholSynthesis methods
The invention provides a novel pyrenyl chalcone derivative and a synthesis method thereof, and belongs to the technical field of organic synthesis. The problem that 3-pyridylaldehyde and 4-pyridylaldehyde cannot react with 1-acetylpyrene under an alkaline or acid condition to obtain chalcone containing pyrenyl and pyridyl is solved. 1-(pyrene-1-yl)-3-(pyridine-3-yl) acrylketone and 1-(pyrene-1-yl)-3-(pyridine-4-yl) acrylketone are obtained through reaction of 1-acetylpyrene, absolute ethyl alcohol and 3-pyridylaldehyde (or 4-pyridylaldehyde) under the action of a catalyst at a certain temperature separately. The catalyst is high in selectivity, the method is simple and convenient, and the yield is relatively high.
Owner:赵明根
Method for synthesizing chalcone derivative with anti-malaria activity
InactiveCN108033951ACheap and easy to buyEasy to operateOrganic chemistryAntiparasitic agentsChalcone derivativeKetone
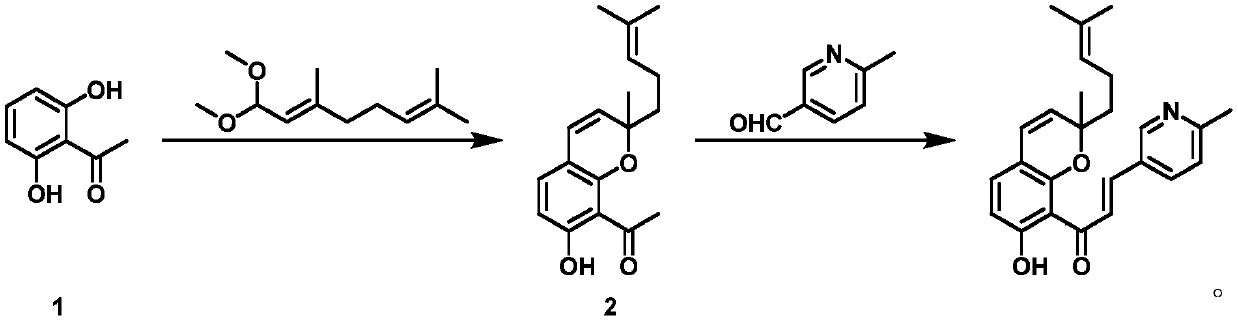
The invention discloses a chalcone derivative (E)-1-(7-hydroxyl-2-methyl-2-(4-methylpentan-1-ol-3-alkene-1-base)-2H-benzopyran-8-base)-3-(6-picoline-3-base) acrylic-2-alkene-1-ketone with anti-malariaactivity and a process for synthesizing the chalcone derivative. The chalcone derivative (E)-1-(7-hydroxyl-2-methyl-2-(4-methylpentan-1-ol-3-alkene-1-base)-2H-benzopyran-8-base)-3-(6-picoline-3-base)acrylic-2-alkene-1-ketone and the process have the advantages that the chalcone derivative is excellent in anti-malaria activity; the chalcone derivative which is a compound can be prepared by the aid of the process which is easy to operate and short in production cycle on a large scale.
Owner:杨文思
A kind of synthetic method of isoliquiritigenin
InactiveCN101353299BSimple methodRaw materials are easy to getCarbonyl compound preparation by condensationCarbonyl compound separation/purificationAcrylophenoneMass spectrometry
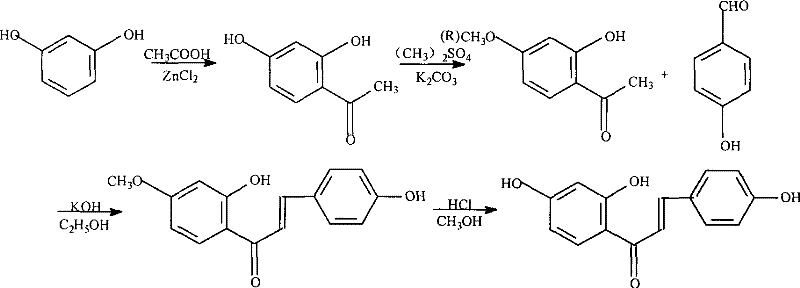
The invention discloses a method for synthesizing chemical substance isoliquiritigenin. The steps are: A. synthesis of 2,4-dihydroxyacetophenone under the catalysis of Lewis base; B. 2-hydroxy-4-methoxyphenethyl Synthesis of ketone; C, Synthesis and recrystallization of 3-(4-hydroxyphenyl)-1-(4-methoxy-2-hydroxyphenyl)-propenone; D, 3-(4-hydroxyphenyl )-1-(2,4-dihydroxyphenyl)-propenone is the synthesis and recrystallization of isoliquiritigenin; E, the crude isoliquiritigenin obtained above is purified by column chromatography to obtain the isoliquiritigenin product, After ultraviolet, infrared, mass spectrometry and nuclear magnetic resonance detection, it is the same as the spectrogram of isoliquiritigenin standard. The invention has the characteristics of simple method, readily available raw materials, low price, high yield and the like.
Owner:WUHAN UNIV
Application of (1,2,3-trimethoxybenzene)-acrylonitrile to preparing medicine for improving senileretinamacular degeneration
ActiveCN109620819ASuppress deathIncreased protectionSenses disorderKetone active ingredientsAntioxidantAcrylonitrile
The invention discloses application of (1,2,3-trimethoxybenzene)-acrylonitrile to preparing medicine for improving senileretinamacular degeneration. The (1,2,3-trimethoxybenzene)-acrylonitrile servesas an antioxidantcapable of activating two-phase enzyme and enhancing the mitochondrial function, has an inhibiting effect on mitochondrial dysfunction caused by acrolein, has an inhibiting effect oncell activity reduction caused by acrolein, and has an inhibiting effect oncell death caused by acrolein. The effect of the (1,2,3-trimethoxybenzene)-acrylonitrile is completed by activating the two-phase enzyme, and the (1,2,3-trimethoxybenzene)-acrylonitrile has wide pharmacological effects of being anticancer, anti-inflammatory, antibacterial and antiviral, treating diabetes, conducting neuroprotection and the like, and is efficient, safe and tidy medicine.
Owner:XI AN JIAOTONG UNIV
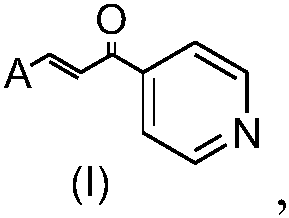
Acrylketone derivative of N-methyl gatifloxacin and preparation method and application of acrylketone derivative
InactiveCN111646974AEffective flatteningSmall toxicityOrganic chemistryAntineoplastic agentsFuranChalcone
The invention belongs to the field of drug synthesis, and relates to a derivative of N-methyl gatifloxacin, in particular to an acrylketone derivative of N-methyl gatifloxacin and a preparation methodand application of the acrylketone derivative of N-methylgatifloxacin. The compound has a structural general formula (I) as shown in the specification, wherein in the formula I, Ar is any one of a benzene ring, a substituted benzene ring, a furan ring or a pyridine ring. According to the acrylketone derivative of N-methyl gatifloxacin, a fluoroquinolone skeleton and an acrylketone skeleton are effectively spliced, so that a novel fluoroquinolone chalcone-like compound is constructed, the anti-tumor activity and the anti-drug resistance of the novel compound are improved, the toxic and side effects on normal cells are reduced, and the novel fluoroquinolone chalcone-like compound can be used as an anti-tumor active substance to develop an anti-tumor drug with a brand-new structure.
Owner:HENAN UNIVERSITY
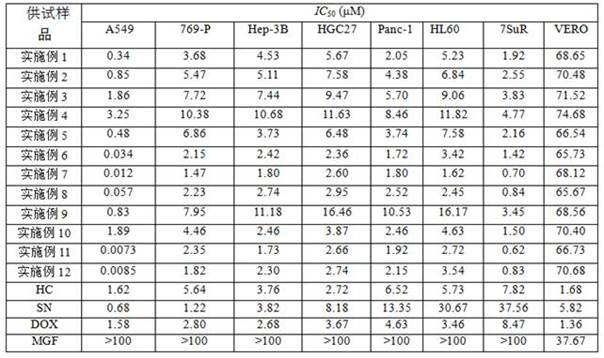
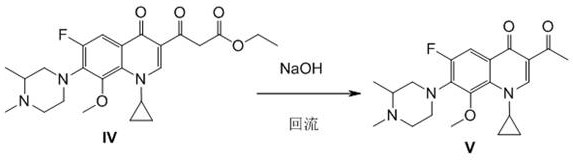
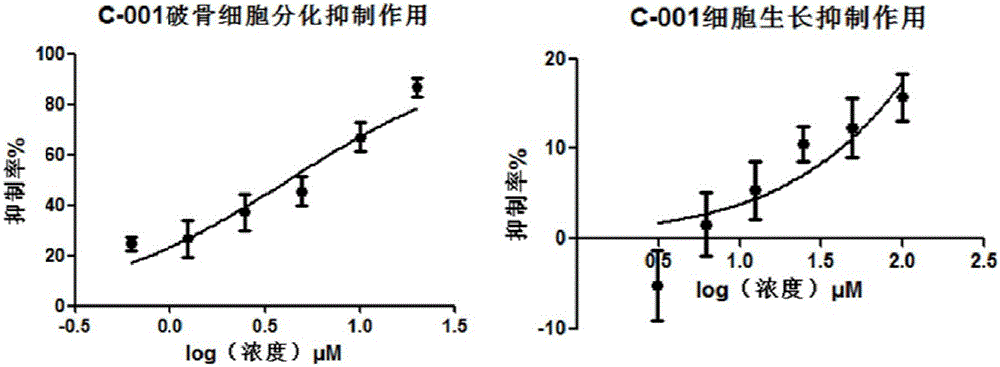
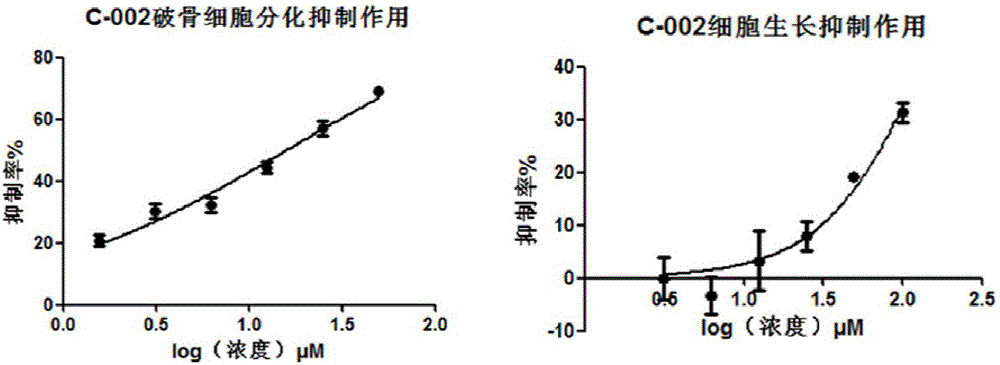
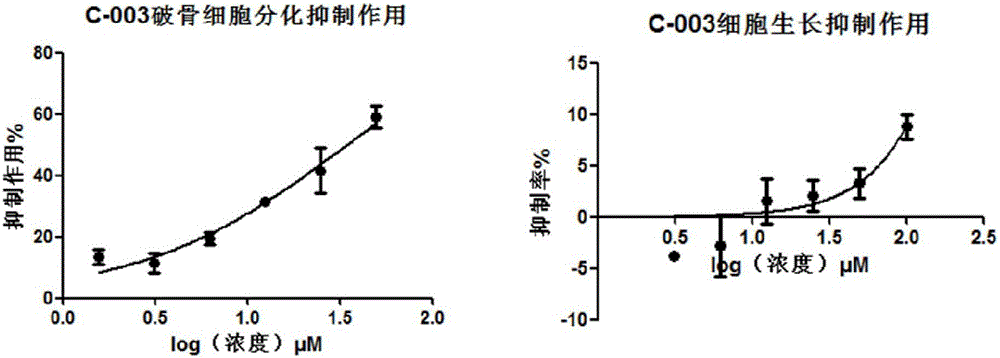
Application of acrylophenone amide derivates to preparation of anti-osteoporosis medicine
ActiveCN106822089AGood differentiation inhibitory activityLow toxicityOrganic active ingredientsSkeletal disorderDiseasePhosphoric acid
The invention relates to medicine application, and specifically discloses application of acrylophenone amide derivates to the preparation of anti-osteoporosis medicine. The structural formula of the acrylophenone amide derivates is shown as formula (I) or formula (II). The compound is different from a molecular skeleton of medicine for preventing and treating osteoporosis used clinically at present, does not belong to a phosphate compound, and is neither estrogen medicine. Cytology experiment researches indicate that the compound can inhibit osteoclast differentiation so as to be of an anti-osteoporosis effect; cytotoxicity tests indicate that the compound is little in toxicity, and can be used for preventing and treating related diseases, particularly osteoporosis, caused by osteoclast prosoplasia.
Owner:GUANGZHOU ZHONGDA NANSHA TECH INNOVATION IND PARK +1
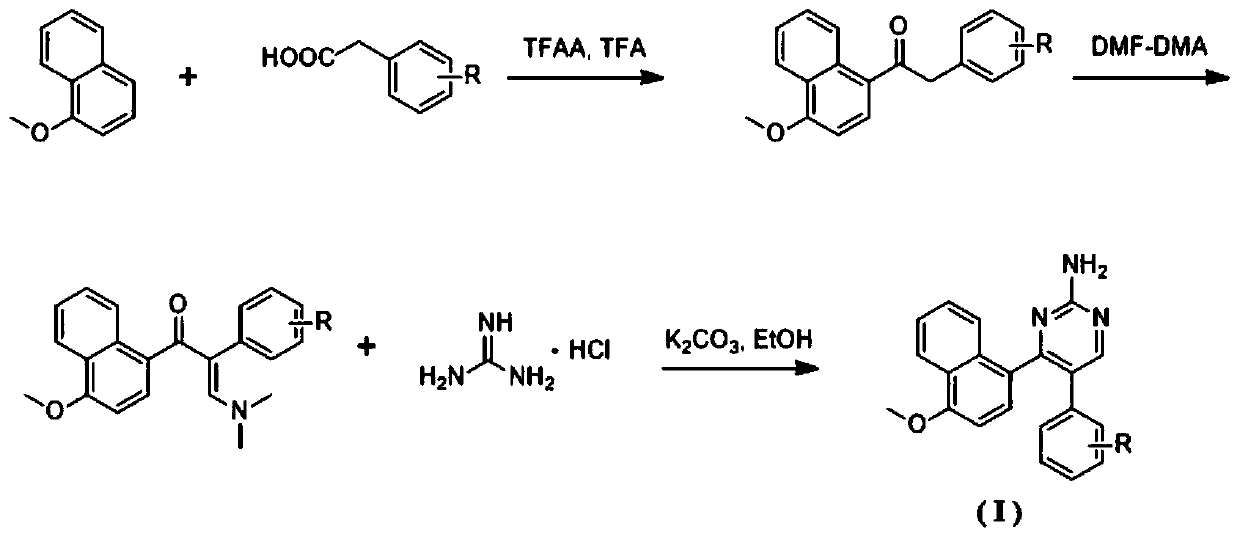
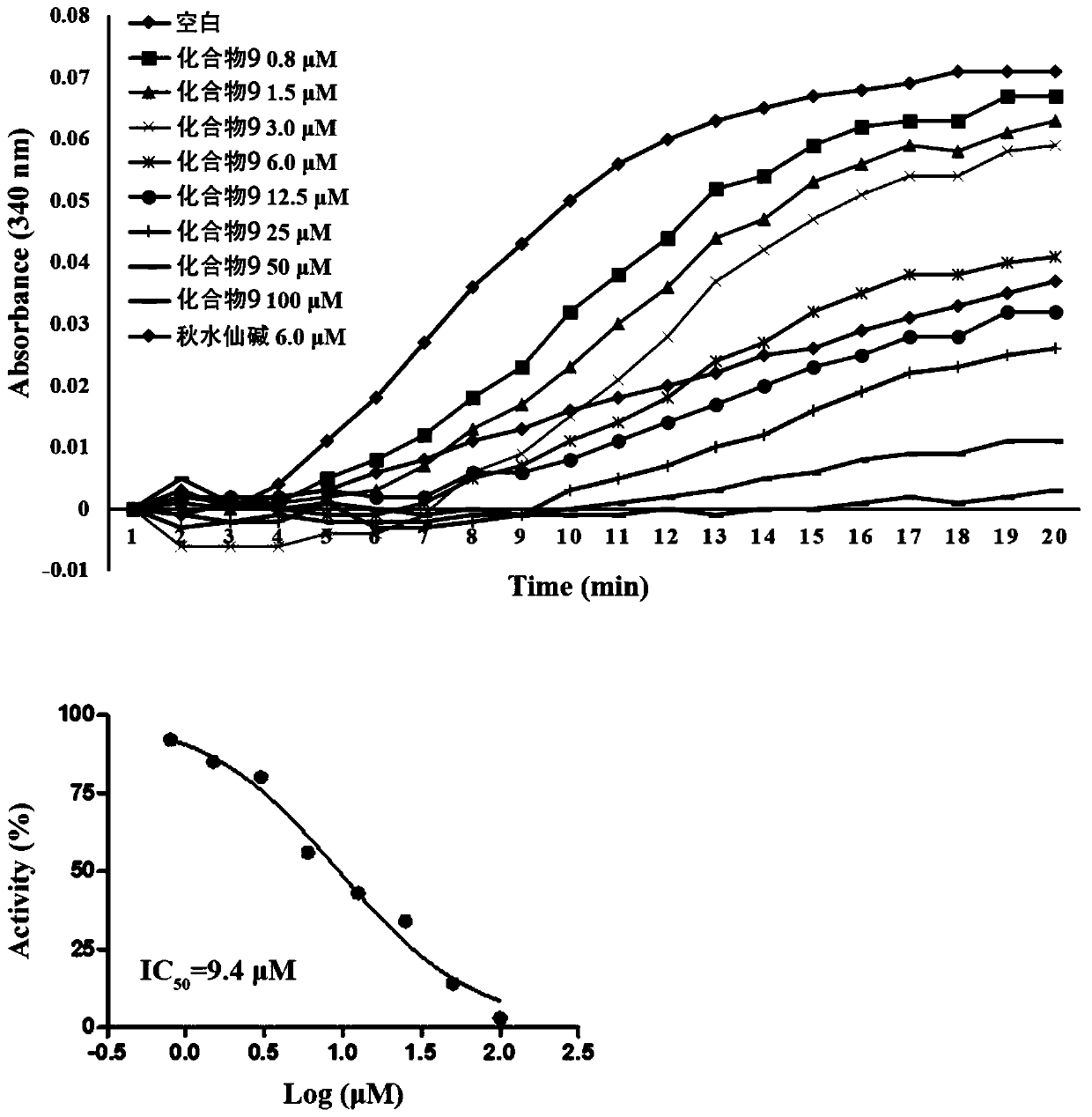
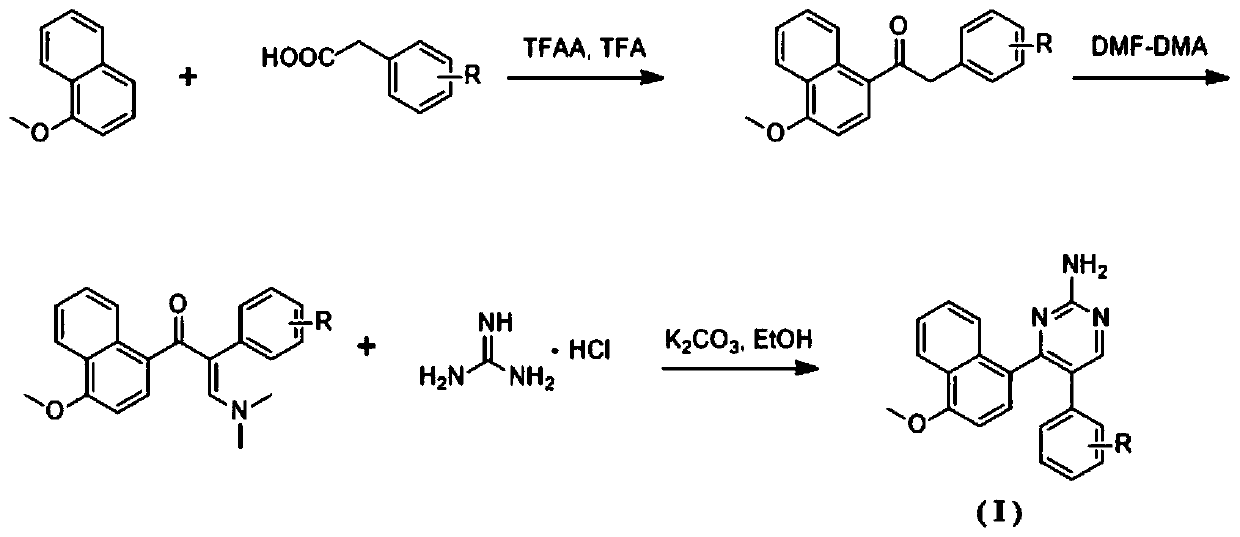
Naphthalene ring-amido pyrimidine type compound and preparation method and application thereof
ActiveCN111138370AEasy to prepareShort synthetic routeOrganic chemistryAntineoplastic agentsPhenylacetic acidHuman breast
The invention discloses a naphthalene ring-aminopyrimidine type compound as well as a preparation method and application thereof, and belongs to the technical field of preparation of new drug compounds. The preparation method disclosed by the invention comprises the following steps: carrying out an acylation reaction between 1-methoxynaphthalene and substituted phenylacetic acid to prepare 1-(4-methoxynaphthalene-1-yl)-2-substituted phenylethane-1-one; carrying out a reaction between 1-(4-methoxynaphthalene-1-yl)-2-substituted phenylethane-1-one and DMF-DMA to prepare (E)-3-(dimethylamino)-1-(4-methoxynaphthalene-1-yl)-2-substituted phenylpropyl-2-ene-1-one, and finally performing a ring closing reaction with guanidine hydrochloride and potassium carbonate to obtain the naphthalene ring-aminopyrimidine compound as shown in the general formular (I) in the specification. The naphthalene ring-amidopyrimidine compound disclosed by the invention has good activity of inhibiting tubulin polymerization, can effectively inhibit the proliferation activity of human breast cancer MCF-7 cells, can be used as a novel lead compound for antitumor drug research, and is suitable for market popularization and application.
Owner:GUIZHOU MEDICAL UNIV
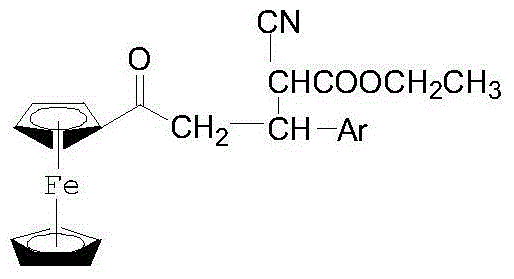
1-Ferrocenyl-aryl-3-(1-cyan-1-ethyl formate-methylene)-acetone and preparation method thereof
The invention discloses 1-ferrocenyl-aryl-3-(1-cyan-1-ethyl formate-methylene)-acetone and a preparation method thereof. The structural formula of the above compound is shown in the specification. The preparation method comprises the following steps: adding 1-ferrocenyl-3-aryl-acrylketone, anhydrous K2CO3 (or NaOH) and ethyl cyanoacetate into a dry mortar, rapidly grinding, detecting the reaction process through TLC, washing with water after the reaction, carrying out pumping filtration, and carrying out vacuum drying, or dissolving by using a small amount of ethyl acetate, carrying out pumping filtration, and concentrating the obtained filtrate until dryness in order to obtain 1-ferrocenyl-aryl-3-(1-cyan-1-ethyl formate-methylene)-acetone. The method has the advantages of small dosage of raw materials, simple operation, mild reaction conditions, simple post-treatment and high yield, and the product prepared in the invention is a brand new ketone, and can be used for sterilization, bacteriostasis, pain easing, inflammation prevention and the like.
Owner:SHAANXI UNIV OF SCI & TECH
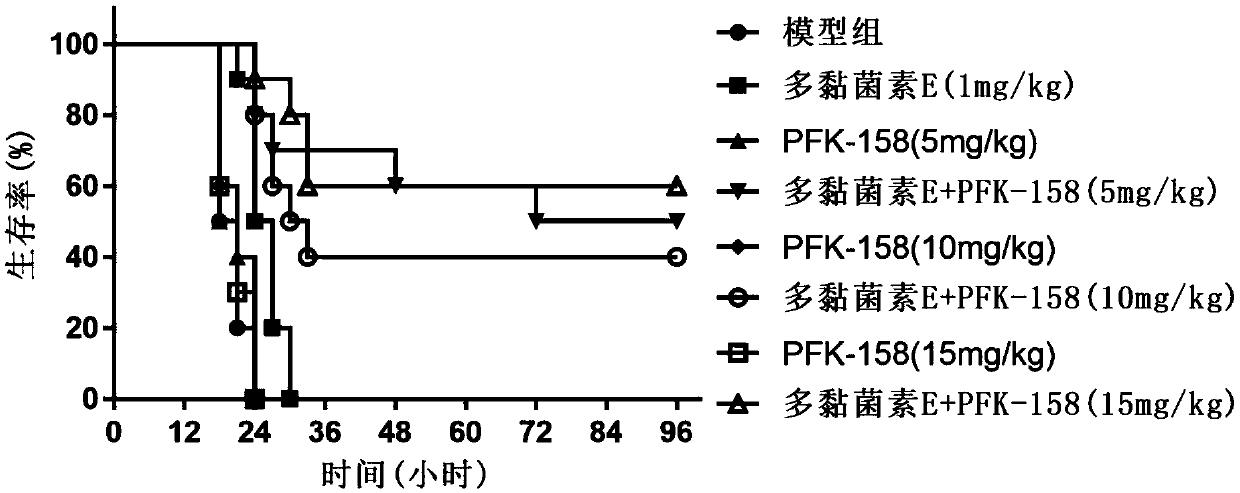
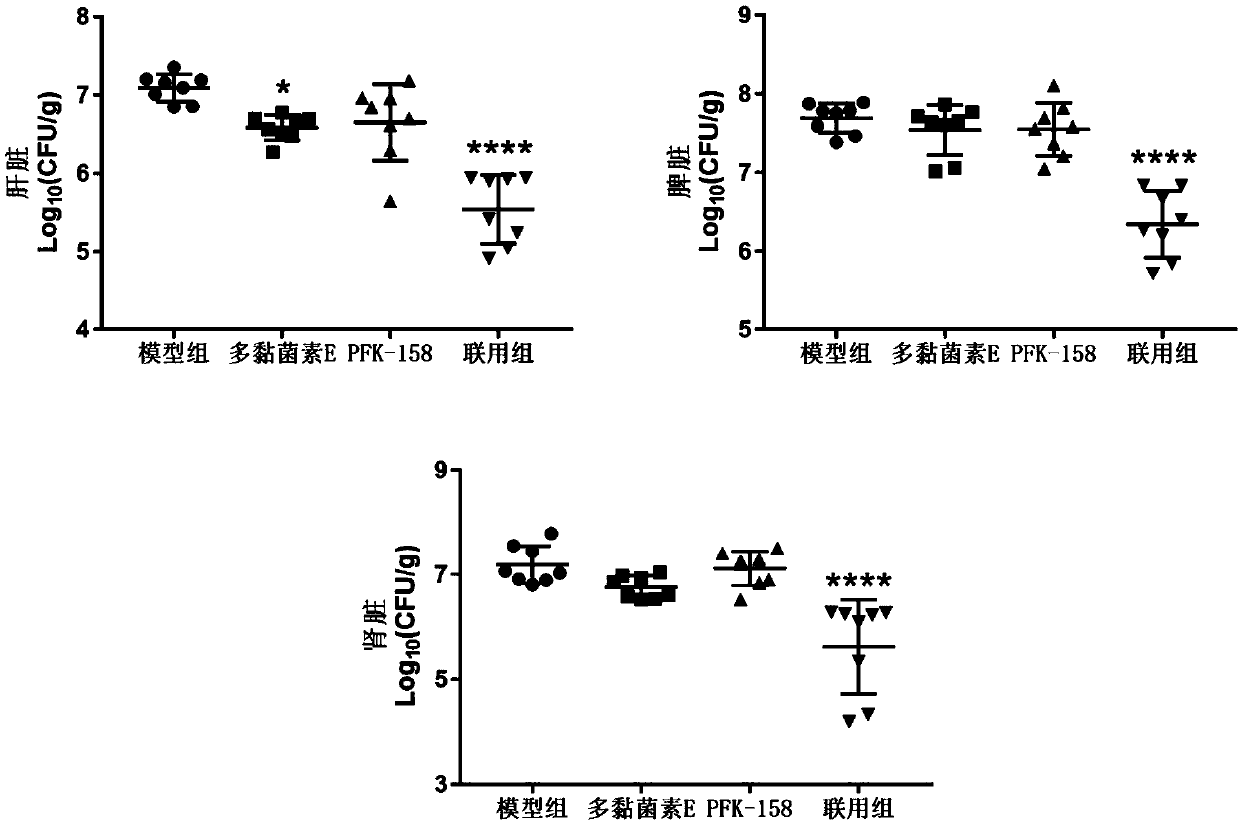
Application of heterocyclic acrylketone type compound as antibacterial agent
ActiveCN109620827AAntibacterial agentsOrganic active ingredientsAntibacterial activityGram-positive bacterium
The invention discloses application of a heterocyclic acrylketone type compound as an antibacterial agent. The compound has remarkable antibacterial activity on gram-positive bacteria; in addition, the heterocyclic acrylketone type compound and polymyxin are combined to have remarkable anti-polymyxin drug-resistance bacterium activity on gram-negative bacteria.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
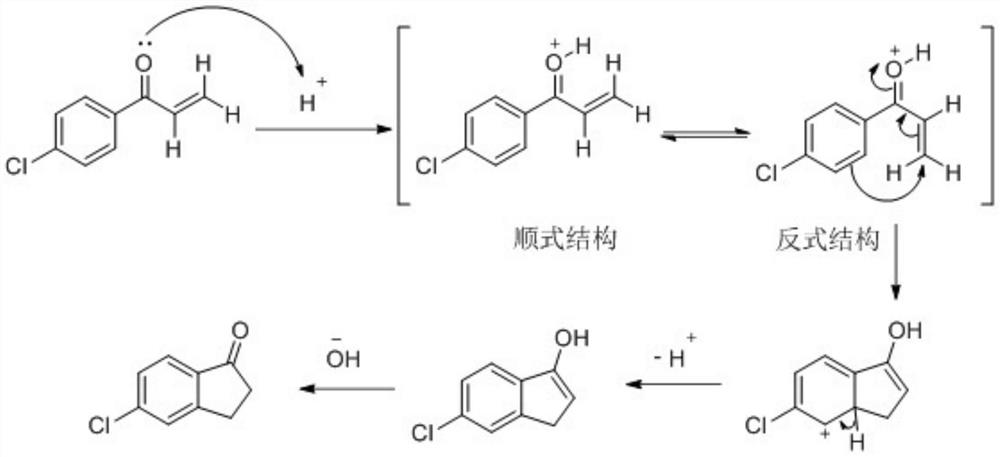
Synthesis method of 5-chloro-1-indanone
ActiveCN113087609AAvoid Explosion HazardsAchieving Intrinsic SafetyOrganic compound preparationCarbonyl compound preparationChlorobenzeneOrganic synthesis
The invention relates to a synthesis method of 5-chloro-1-indanone, and belongs to the technical field of organic synthesis. According to the invention, 1-(4-chlorphenyl)-2-propylene-1-ketone is used as a raw material, and under the catalysis of hydrogen chloride, intramolecular cyclization is carried out to generate 5-chloro-1-indanone. The hydrogen chloride gas is used for replacing aluminum trichloride catalysis in a traditional process, the low-temperature process is used for replacing the high-temperature process, the risk that aluminum trichloride possibly explodes when encountering water under the high-temperature condition is avoided, and essential safety of the process is achieved. Besides, the synthesis method only generates a small amount of acid-base neutralization wastewater, so that the environmental protection benefit is greatly improved.
Owner:山东京博生物科技有限公司
Acrylketone derivative of N-methyl lomefloxacin and preparation method and application of acrylketone derivative
ActiveCN111646975AGood anti-tumor effectTo achieve synergistic effect and detoxificationOrganic chemistryAntineoplastic agentsFuranLomenfloxacin
The invention belongs to the field of drug synthesis, and relates to a derivative of N-methyl lomefloxacin, in particular to an acrylketone derivative of N-methyllomefloxacin and a preparation methodand application of the acrylketone derivative. The acrylketone derivative of N-methyllomefloxacin has the following structural general formula (I): in the formula, Ar is selected from any one of a benzene ring, a furan ring or a pyridine ring and a substituent group thereof. According to the acrylketone derivative of N-methyl lomefloxacin, a fluoroquinolinone skeleton and an acrylketone skeleton are effectively spliced, so that a novel fluoroquinolinone chalcone-like compound is constructed, the antitumor activity and the drug resistance of the novel compound are improved, the toxic and side effects on normal cells are reduced, and the fluoroquinolinone chalcone-like compound can be used as an antitumor active substance to develop antitumor drugs with brand new structures.
Owner:HENAN UNIVERSITY
1-ferrocenyl-3-aryl-3-cyano-methylene-acetone and preparation method thereof
InactiveCN104478944AHigh yieldRaw materials are easy to getFungicidesDisinfectantsArylAfter treatment
The invention discloses 1-ferrocenyl-3-aryl-3-cyano-methylene-acetone and a preparation method thereof. 1-ferrocenyl-3-aryl-3-cyano-methylene-acetone has a structural formula described in the description. The preparation method comprises the following steps: adding A mol of 1-ferrocenyl-3-aryl-acrylketone, B mol of anhydrous K2CO3 (or NaOH) and C mol of acetonitrile into a dried mortar, quickly grinding, performing TLC (Thin Layer Chromatography) detection until complete reaction, washing with water, performing suction filtration, and drying to obtain 1-ferrocenyl-3-aryl-3-cyano-methylene-acetone. The preparation method has the advantages of being short in reaction time, free of solvent, environment friendly, good in economic property, simple in operation, mild in reaction conditions, simple in after-treatment and high in yield.
Owner:SHAANXI UNIV OF SCI & TECH
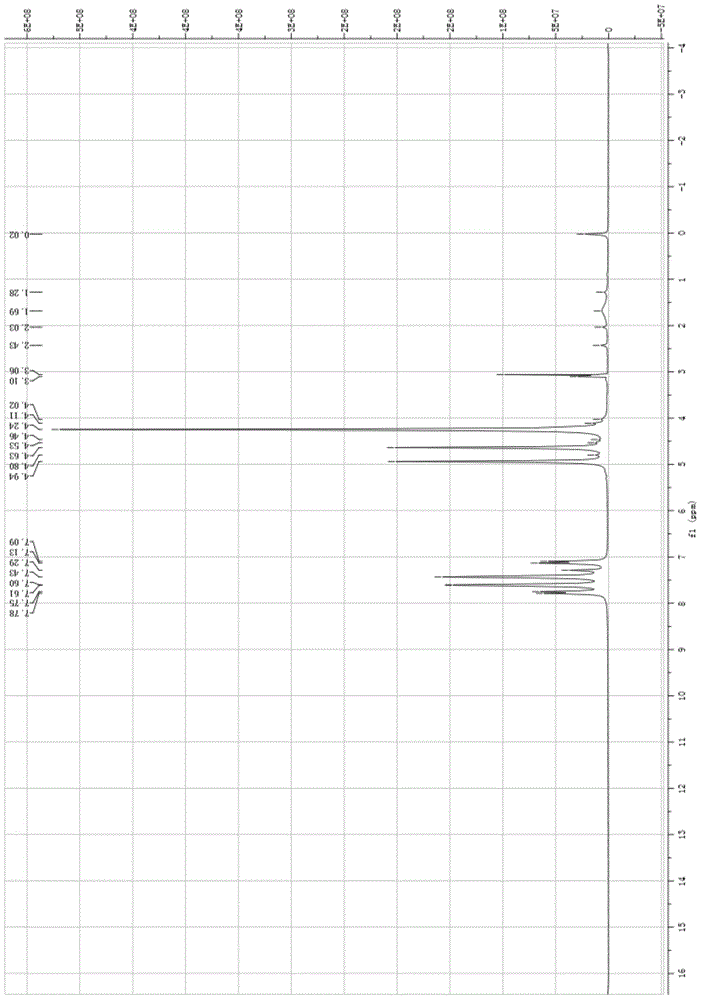
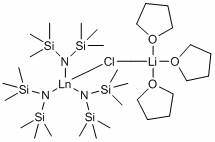
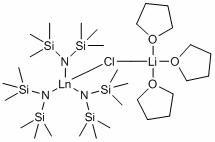
Application of silylamino rare earth compound in catalysis of reaction of isatin compound and cyclopropenone compound
PendingCN112958154AHigh yieldRaw materials are easy to obtainOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCombinatorial chemistryAcrylophenone
The invention discloses application of ansilylamino rare earth compound in catalysis of a reaction of an isatin compound and a cyclopropenone compound. The silylamino rare earth compound is [(Me<3>Si)<2>N]<3>Ln(m-Cl)Li(THF)<3>. In the presence of phosphite, a reaction of the isatin compound and cyclopropenone is catalyzed by thesilylaminorare earth compound, so a method, which is simple in raw material source, simple in step, mild in reaction conditions, high in activity and good in universality, for synthesizing the pyrano[2,3-b]indol-2-one compound,is realized.
Owner:SUZHOU UNIV
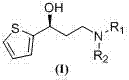
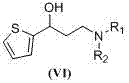
Preparation method of (S)-3-N,N-disubstituted amino-1-(2-thienyl)-1-propanol
InactiveCN107488163AMild reaction conditionsThree wastes lessOptically-active compound separationOrganic racemisationOrganic base1-Propanol
The invention discloses a preparation method of (S)-3-N,N-disubstituted amino-1-(2-thienyl)-1-propanol shown as the formula (I). The method comprises: taking 2-acetyl thiophene shown in the formula (II) as a raw material, and allowing 2-acetyl thiophene to completely react with di(trichloromethyl)carbonic ester (III) and N,N-disubstituted methanamide (IV) in an organic solvent under the catalysis of an organic base to obtain N,N-disubstituted amino-1-(2-thienyl)-1-acrylketone shown as the formula (V); and performing hydrogenation reduction with lithium aluminium hydride to obtain N,N-disubstituted amino-1-(2-thienyl)-1-propanol shown as the formula (VI); and performing splitting with S-mandelic acid and recrystallization with ethyl acetate to obtain the target product shown as the formula (I). The preparation method is low in cost, mild in reaction condition, less in waste water, waste gas and industrial residue, small in energy consumption, and high in yield. The preparation method is safe and is suitable for industrial production.
Owner:天台宜生生化科技有限公司
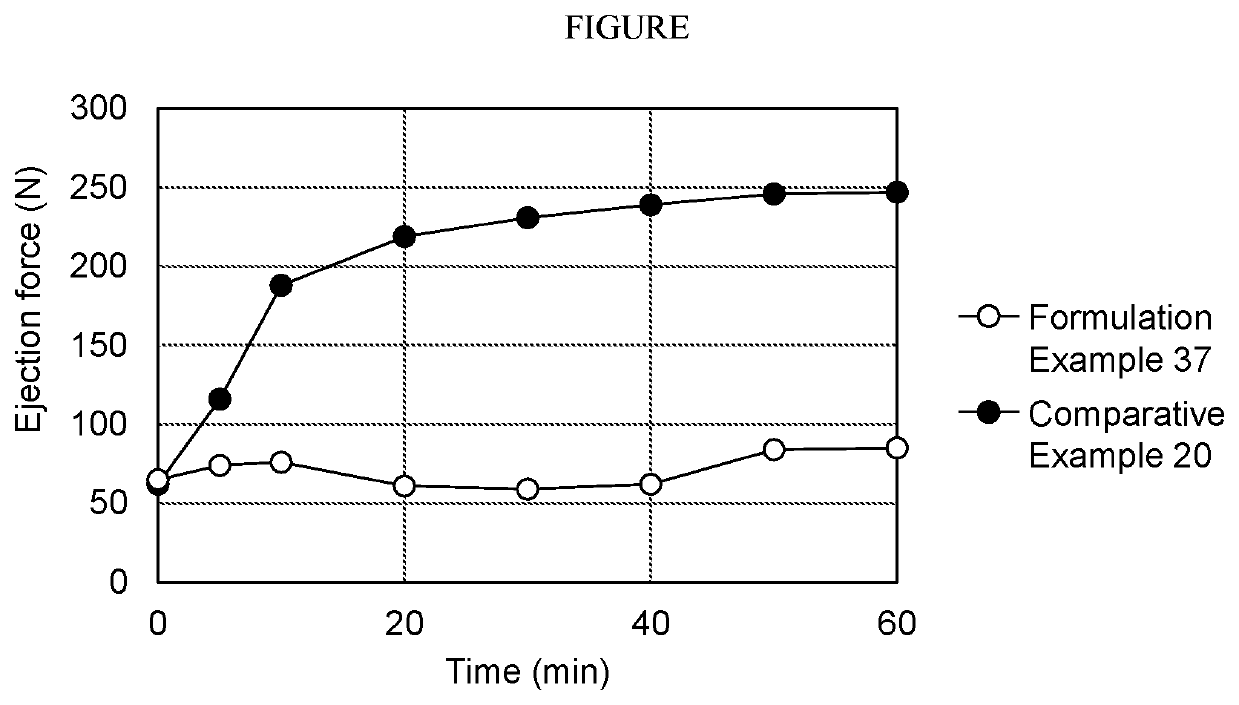
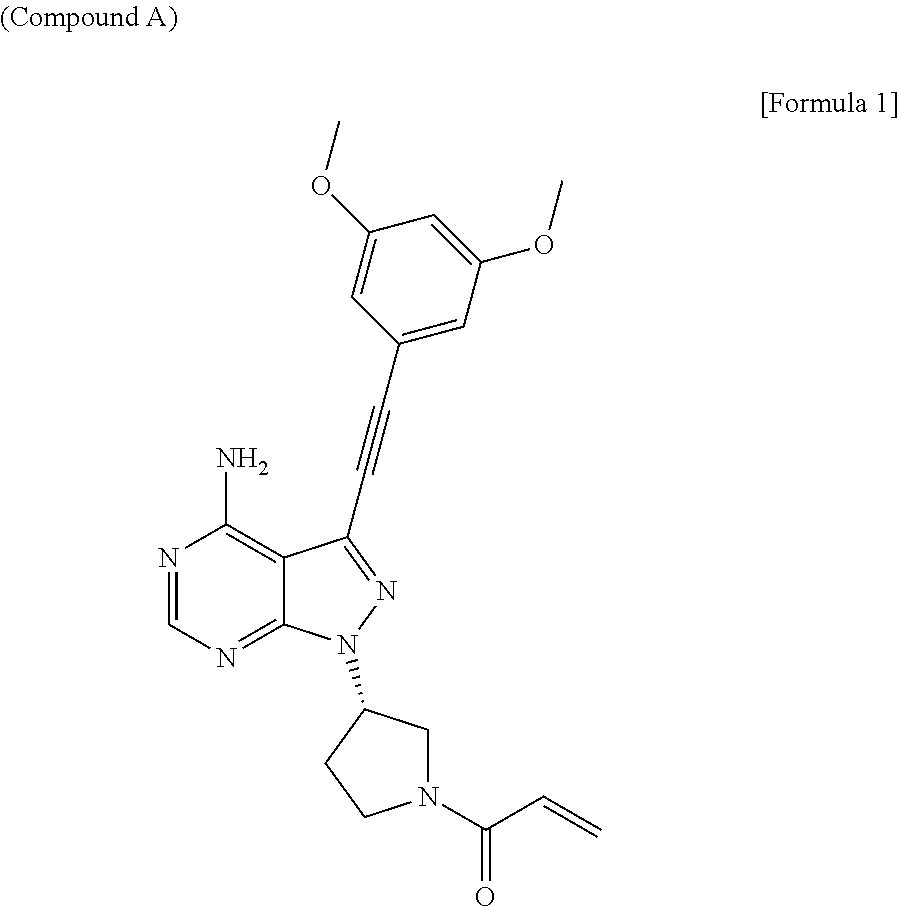
Pharmaceutical composition including sodium alkyl sulfate
PendingUS20210030755A1Improve solubilityImprove stabilityPowder deliveryDispersion deliveryPharmaceutical drugPyrrolidine
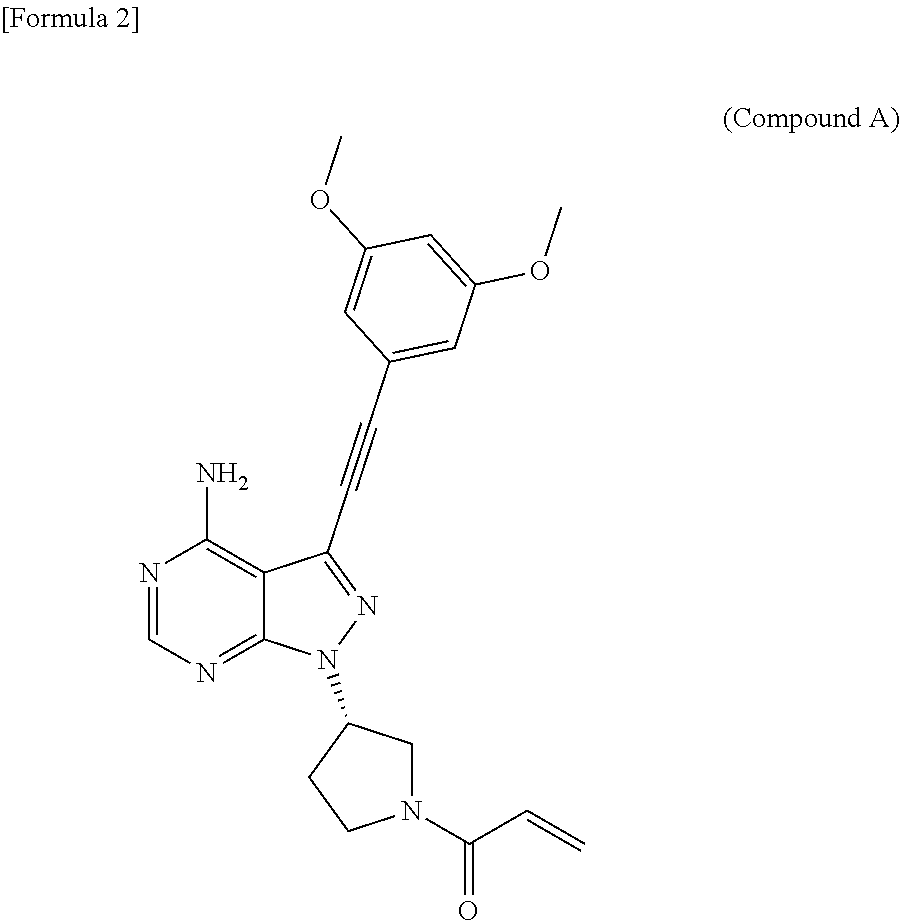
The object of the present invention is to improve the dissolution and the absorption of (S)-1-(3-(4-amino-3-((3,5-dimethoxyphenyl)ethynyl-1H-pyrazolo[3,4-d]pyrimidin-1-yl-1-pyrrolidinyl)-2-propen-1-one effective as an antitumor agent from a pharmaceutical formulation comprising the same. Provided is a pharmaceutical composition comprising (S)-1-(3-(4-amino-3-((3,5-dimethoxyphenyl)ethynyl-1H-pyrazolo[3,4-d]pyrimidin-1-yl-1-pyrrolidinyl)-2-propen-1-one in combination with sodium alkyl sulfate having an alkyl group containing 10 to 18 carbon atoms, in particular, with sodium lauryl sulfate.
Owner:TAIHO PHARMA CO LTD
Preparation method of 1, 2-dihydrocyclopenta [b] chromene-3, 9-diketone compound
The invention provides a preparation method of a 1, 2-dihydrocyclopenta [b] chromene-3, 9-diketone compound, and relates to the technical field of chemistry. According to the preparation method of the 1, 2-dihydrocyclopenta [b] chromene-3, 9-diketone compound, 3-iodo chromone, norbornene and cyclopropenone are taken as raw materials, and a [2 + 2 + 1] series cyclization reaction is carried out in an organic solvent under the condition of a palladium catalyst, so a series of 1, 2-dihydrocyclopenta [b] chromene-3, 9-diketone compounds are synthesized; the compounds contain a chromone skeleton with potential biological activity, can provide a compound source for biological activity screening, and have important application value in the field of medicinal chemistry. Meanwhile, cyclopropenone is used as a unique carbonyl source to participate in the palladium-catalyzed carbonylation reaction for the first time, and a new method is provided for the palladium-catalyzed carbonylation reaction for synthesizing carbonyl compounds.
Owner:ZUNYI MEDICAL UNIVERSITY
1,2-diaryl-2-propenyl-1-ketone compounds and use thereof
InactiveCN103183598AConvenient treatmentOrganic compound preparationKetone active ingredientsArylKetone
The invention belongs to the technical field of medicines, relates to 1,2-diaryl-2-propenyl-1-ketone compounds and use thereof, and particularly relates to the compounds and application thereof as a tumor cell proliferation inhibitor and a tumor vascular disrupting agent in field of preparation of anti-cancer medicines. The compounds of the present invention can be represented by a structural formula in the specification, wherein R1-R8 are defined as the specification.
Owner:SHENYANG PHARMA UNIVERSITY
Acrylketone derivative without N-methyl ofloxacin and preparation method and application thereof
ActiveCN111647004ATo achieve synergistic effect and detoxificationTo achieve the effect of anti-drug resistanceOrganic chemistryAntineoplastic agentsSide effectChalcone
The invention belongs to the field of drug synthesis, and relates to a derivative without N-methyl ofloxacin, in particular to an acrylketone derivative without N-methyl ofloxacin and a preparation method and application thereof. The compound has a structural general formula (I) as shown in the specification, wherein in the formula I, Ar is any one of a benzene ring, a substituted benzene ring, afuran ring or a pyridine ring. According to the acrylketone derivative without N-methyl ofloxacin and the preparation method thereof, a fluoroquinolinone skeleton and an acrylketone skeleton are effectively spliced, so that a novel fluoroquinolinone chalcone-like compound is constructed, the antitumor activity and the drug resistance of the novel compound are improved, the toxic and side effects on normal cells are reduced, and the fluoroquinolinone chalcone-like compound can be used as an antitumor active substance to develop antitumor drugs with brand new structures.
Owner:HENAN UNIVERSITY +1
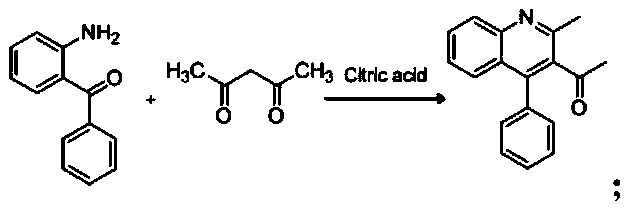
Preparation method and application of chalcone derivative QNL-Chalcone
The invention relates to the field of drug synthesis, and discloses a preparation method and application of a novel chalcone derivative QNL-Chalcone aiming at the problems of insufficient molecular activity and long synthesis route in the prior art. The preparation method comprises the following steps: (1) synthesis of 1-(2-methyl-4-phenylquinoline-3-yl) ethanone; and (2) synthesis of QNL-Chalcone; wherein acetylacetonamine, 2-aminobenzophenone and 2-bromobenzaldehyde are taken as raw materials, and QNL-Chalcone is prepared through the above two preparation steps. According to the invention, quinoline parent nucleuses are introduced, and a chalcone activity essential group: acrylketone structure is reserved, so that the compound QNL-Chalcone with strong anti-depression activity and low side effect is obtained; the synthetic route of the medicine is short, the cost is low, the process is good in controllability and easy for actual large-scale industrial mass production, and the preparedproduct is high in yield and purity.
Owner:ZHEJIANG OCEAN UNIV
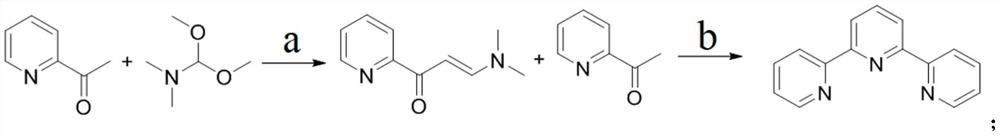
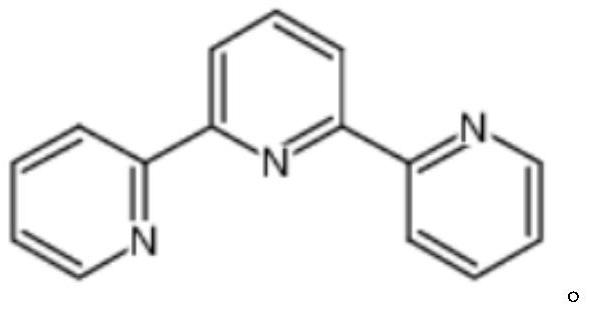
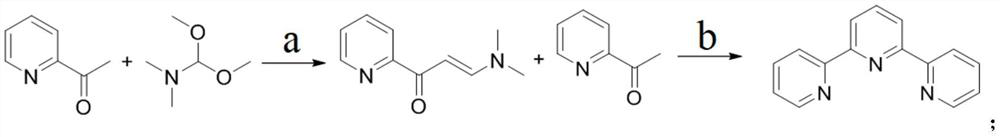
Method for large-scale production of alpha, alpha, alpha-terpyridyl
ActiveCN113354573AHigh purityMild reaction conditionsOrganic chemistryBulk chemical productionPtru catalystDimethyl acetal
The invention discloses a method for large-scale production of alpha, alpha, alpha-terpyridyl. The method adopts the following synthetic route: the reaction operation in the step a is as follows: adding 2-acetylpyridine, N, N-dimethylformamide dimethyl acetal and a catalyst into an organic solvent at room temperature, reacting under reflux, and after the reaction is finished, after cooling the reaction solution to room temperature, pouring the reaction solution into a sodium hydroxide aqueous solution, extracting a product by using dichloromethane, washing, drying and concentrating an obtained organic phase, and recrystallizing an obtained crude product to obtain an intermediate, namely 1-(3-pyridyl)-3-(dimethylamino)-2-propylene-1-ketone. The method not only is easy to realize large-scale production, but also is high in yield, low in cost, simple to operate and mild in reaction condition.
Owner:河南阿尔法医药科技有限公司
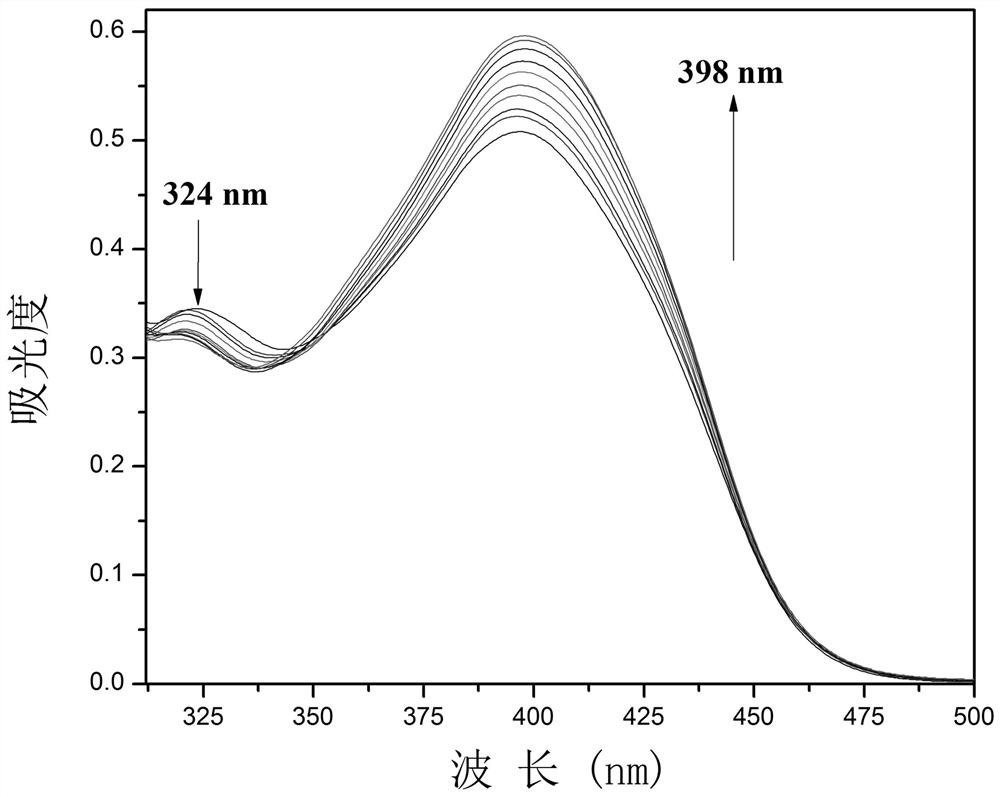
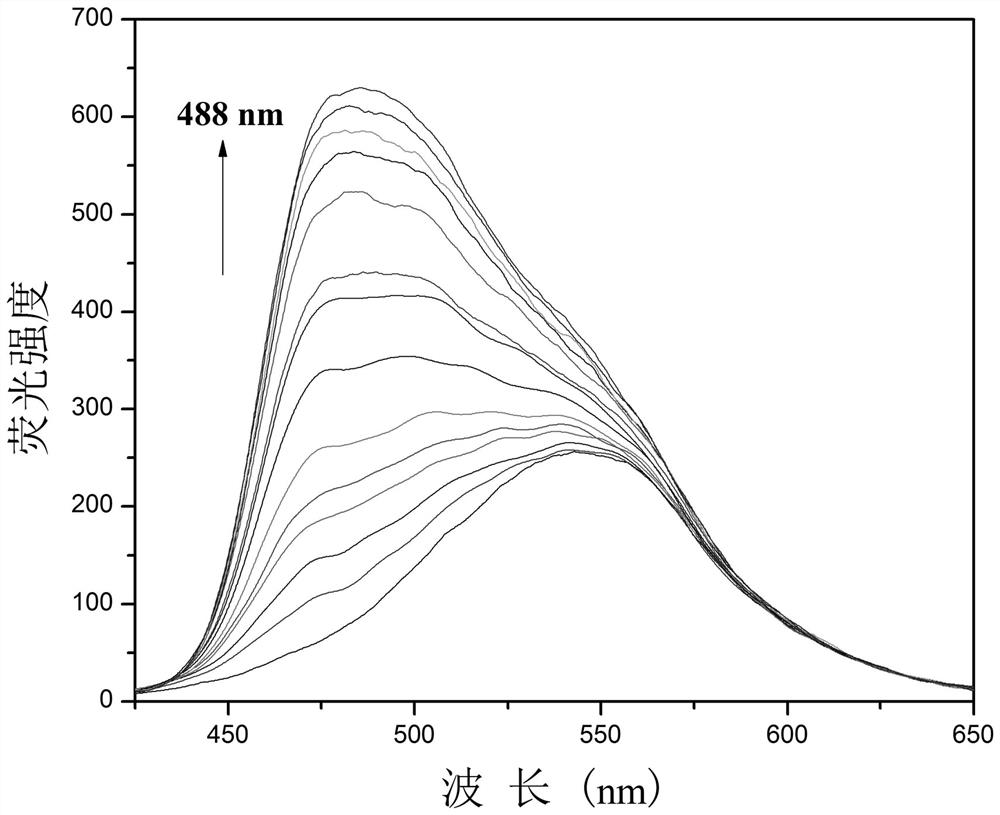
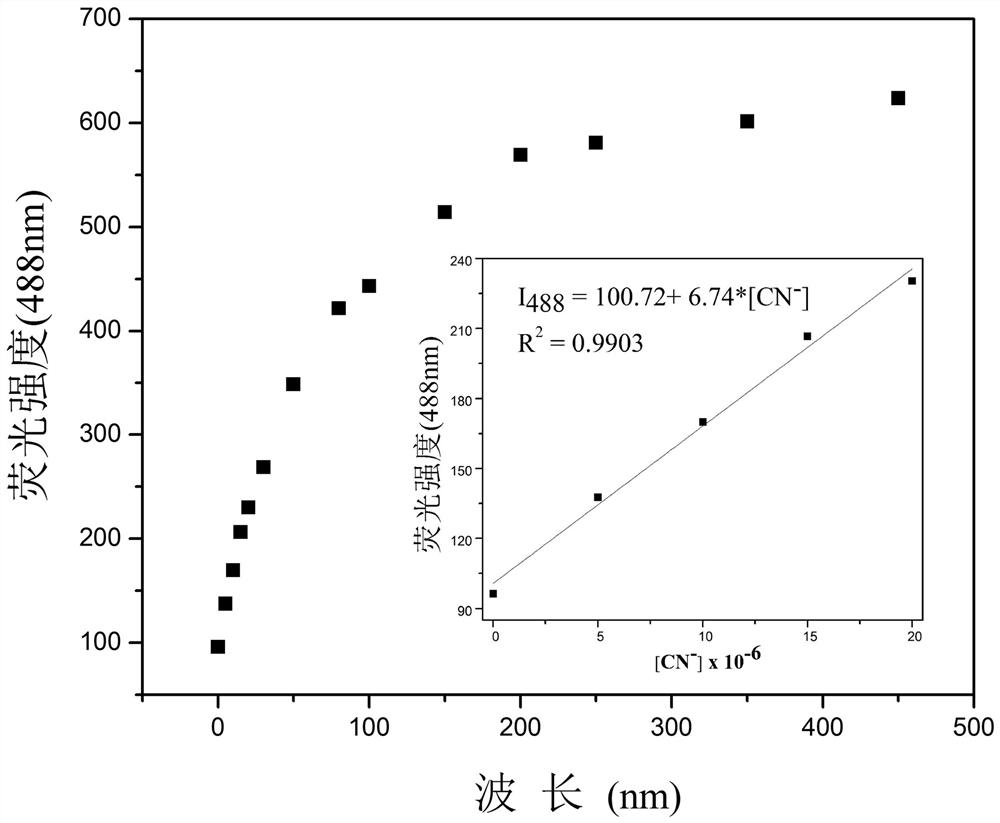
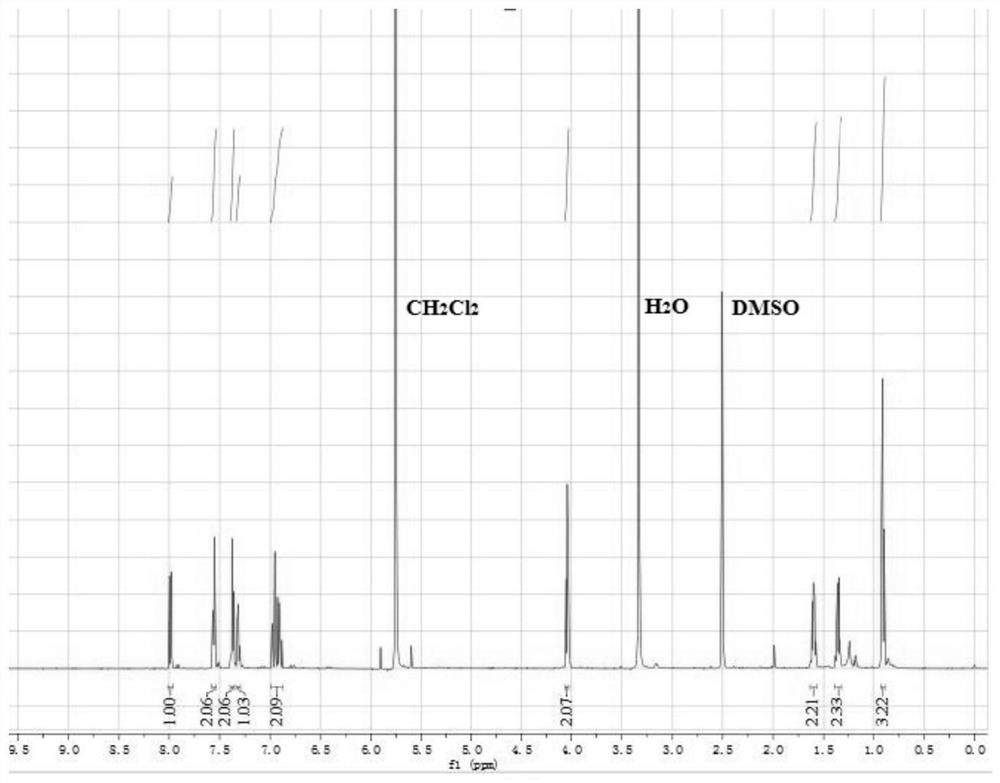
One for detection of cn - Enhanced fluorescent probe and its preparation method and biological application
ActiveCN109232379BThe synthesis steps are simpleLow costOrganic chemistryFluorescence/phosphorescenceFluoProbesFluorescence
Owner:SHANXI UNIV
A kind of preparation method and application of 3-phenyl-2-propen-1-one o-n-butyl oxime
ActiveCN113354557BGuaranteed supplyReduce manufacturing costHydrocarbon purification/separationHydrocarbonsHydroxylamineOrganic synthesis
A 3‑phenyl‑2‑propen‑1‑one O A preparation method for n-butyl oxime, which belongs to the technical field of organic synthesis. The method uses 3-phenyl-2-acrolein, hydroxylamine hydrochloride and brominated n-butane as raw materials, and is prepared under potassium hydroxide alkaline conditions. The crude product obtained from the reaction obtains 3-phenyl-2-propene-1-ketone through flash column chromatography O ‑n-Butyl oxime. Compound 3‑phenyl‑2‑propen‑1‑one O ‑n-Butyl oxime can be used as a new type of polymerization inhibitor, especially for styrene. Adopt method of the present invention to prepare 3-phenyl-2-propene-1-ketone under conventional conditions O ‑n-Butyl oxime, safe and simple to operate, saving energy. The starting material of the method is cheap, which is beneficial to reduce the production cost. The process is simple, the operation is convenient, and the conversion rate of raw materials is high.
Owner:DALIAN UNIV OF TECH
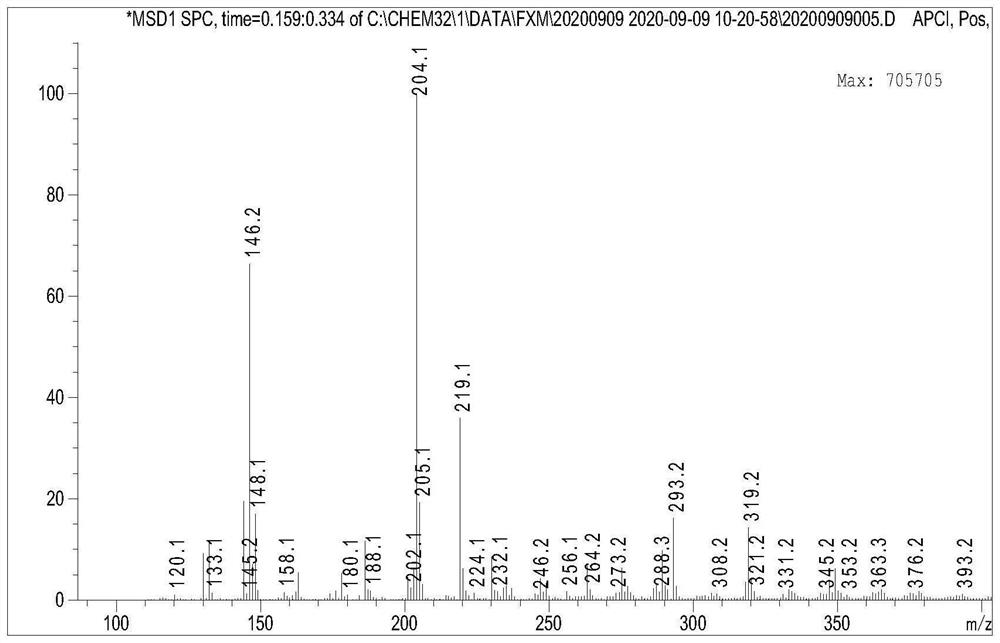
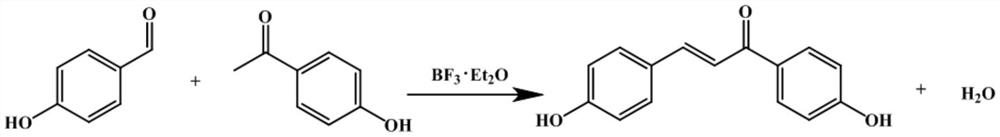
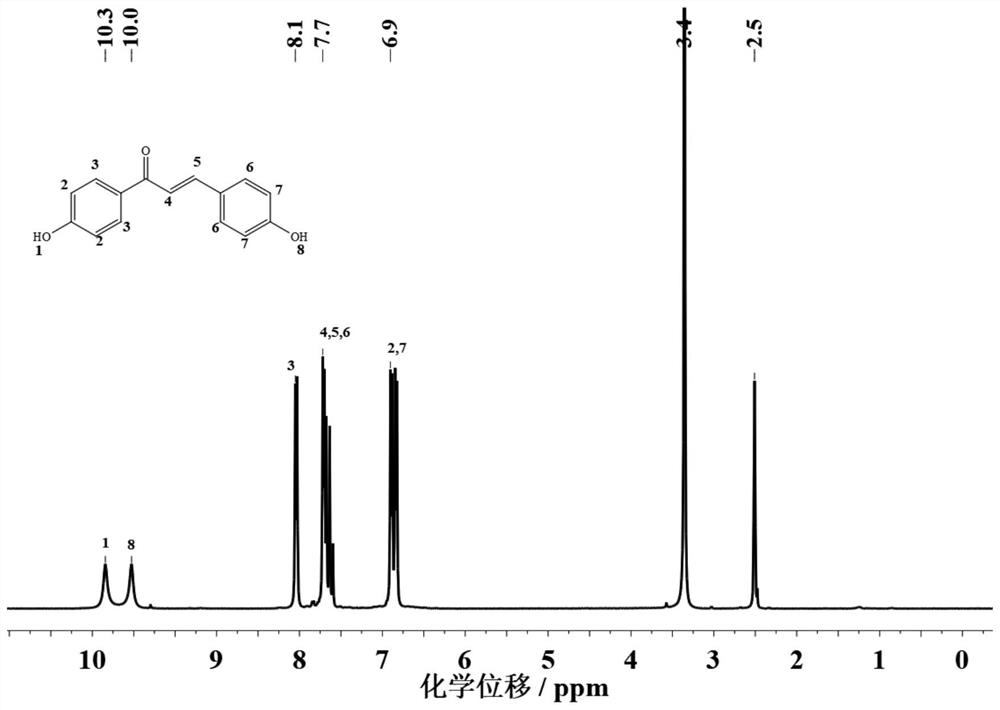
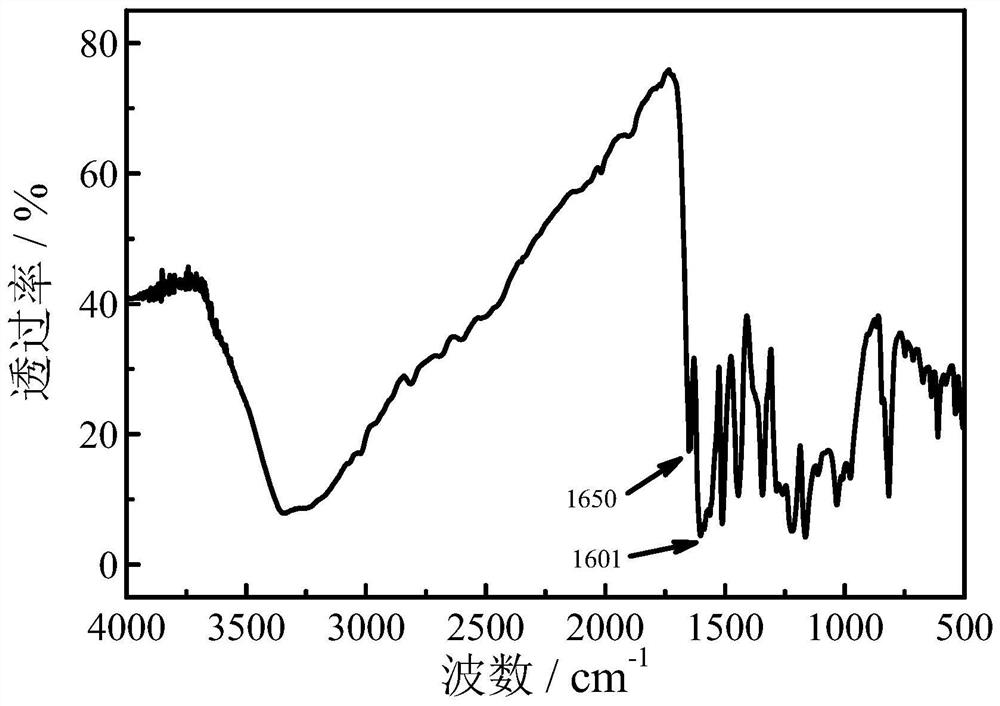
A method for efficiently synthesizing 1,3-bis(4-hydroxyphenyl)-2-propen-1-one
ActiveCN112239401BKeep aliveEfficient synthesisOrganic compound preparationCarbonyl compound preparationChemical synthesisSchmidt reaction
The invention discloses a method for efficiently synthesizing 1,3-bis(4-hydroxyphenyl)-2-propene-1-one, which belongs to the field of organic chemical synthesis. The method is through the Claisen-Schmidt reaction, with p-hydroxybenzaldehyde and p-hydroxyacetophenone as reactants, and boron trifluoride ether as a catalyst, by gradually adding the catalyst to the raw material solution, and adding dry Agents can effectively ensure the activity of the catalyst, thereby optimizing the reaction to obtain the product. After the reaction, the product solution was added dropwise into deionized water, and the precipitate was collected and dried. This method is convenient and quick, easy to implement, can realize the synthesis of 1,5-bis(4-hydroxyphenyl)-1,4-pentadien-3-one with high yield (>98%), and has important practical application value , suitable for industrial production.
Owner:NANCHANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



![Preparation method of 1, 2-dihydrocyclopenta [b] chromene-3, 9-diketone compound Preparation method of 1, 2-dihydrocyclopenta [b] chromene-3, 9-diketone compound](https://images-eureka.patsnap.com/patent_img/be807399-0c9f-4c13-acaa-2e1a88e7dbda/210129163739.png)
![Preparation method of 1, 2-dihydrocyclopenta [b] chromene-3, 9-diketone compound Preparation method of 1, 2-dihydrocyclopenta [b] chromene-3, 9-diketone compound](https://images-eureka.patsnap.com/patent_img/be807399-0c9f-4c13-acaa-2e1a88e7dbda/210129163742.png)
![Preparation method of 1, 2-dihydrocyclopenta [b] chromene-3, 9-diketone compound Preparation method of 1, 2-dihydrocyclopenta [b] chromene-3, 9-diketone compound](https://images-eureka.patsnap.com/patent_img/be807399-0c9f-4c13-acaa-2e1a88e7dbda/210129163747.png)