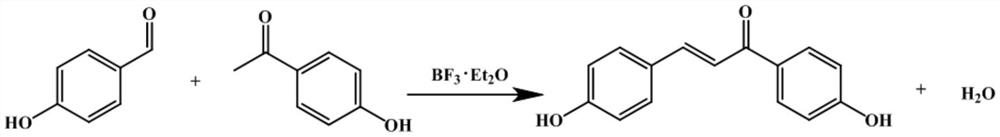
A method for efficiently synthesizing 1,3-bis(4-hydroxyphenyl)-2-propen-1-one
A technology for hydroxyphenyl and p-hydroxyacetophenone, applied in the field of efficient synthesis of 1,3-di-2-propen-1-one, which can solve the problems of complex time-consuming, long reaction time, and low yield , to achieve the effect of solving low yield, ensuring activity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 1.22g (10mmol) p-hydroxybenzaldehyde, 1.77g (13mmol) p-hydroxyacetophenone, 6g 4A type molecular sieve into the round bottom flask, add 10mL 1,4-dioxane, stir to dissolve the reactant Add 0.25 mL of boron trifluoride ether; dissolve 1 mL of boron trifluoride ether in 4 mL of dioxane, and inhale it into a syringe without a rubber tip; fix the syringe on the syringe pump and adjust the appropriate rate so that trifluoride Boronium ether solution was injected into the substrate solution within 5 hours at the same time, while stirring and heating to 80°C for reaction, after the injection was completed, the reaction was continued for 10 hours, cooled to room temperature, and then the product solution was added dropwise to 400mL deionized water, The wine-red powder precipitate was obtained by suction filtration, and the pure product was obtained by vacuum drying at 80° C. for 10 h, with a yield of 98%.
Embodiment 2
[0027] 1.46g (12mmol) p-hydroxybenzaldehyde, 1.36g (10mmol) p-hydroxyacetophenone, 6g anhydrous MgSO 4 Put it into a round bottom flask, add 5mL of methanol, stir to dissolve the reactant, then add 0.3mL of boron trifluoride ether; dissolve 0.8mL of boron trifluoride ether in 2.2mL of ethanol, and seal it well. The boron trifluoride ether solution was added dropwise to the substrate solution within 3 hours at the same time, and started to stir and heat to 80°C to react at the same time. After the injection was completed, the reaction was continued for 5 hours and cooled to room temperature, and then the product solution was added dropwise to 300mL Suction filter in deionized water to obtain a wine red powder precipitate, and vacuum dry at 80°C for 10 hours to obtain a pure product with a yield of 99%.
Embodiment 3
[0029] 1.22g (10mmol) p-hydroxybenzaldehyde, 1.36g (10mmol) p-hydroxyacetophenone, 6g anhydrous NaSO 4 Put it into a round bottom flask, add 5mL of ethanol, stir to dissolve the reactant, then add 0.2mL of boron trifluoride ether; dissolve 1.5mL of boron trifluoride ether in 8.8mL of ethanol, and use a dropping funnel to make the The boron ether solution was added dropwise to the substrate solution at the same time within 2 hours. While adding, stir and heat to 60°C for reaction. After the injection, continue to react for 5 hours and cool down to room temperature, then add the product solution dropwise into 500mL deionized water , suction filtration to obtain a wine red powder precipitate, and vacuum drying at 80°C for 10 hours to obtain a pure product with a yield of 96%.
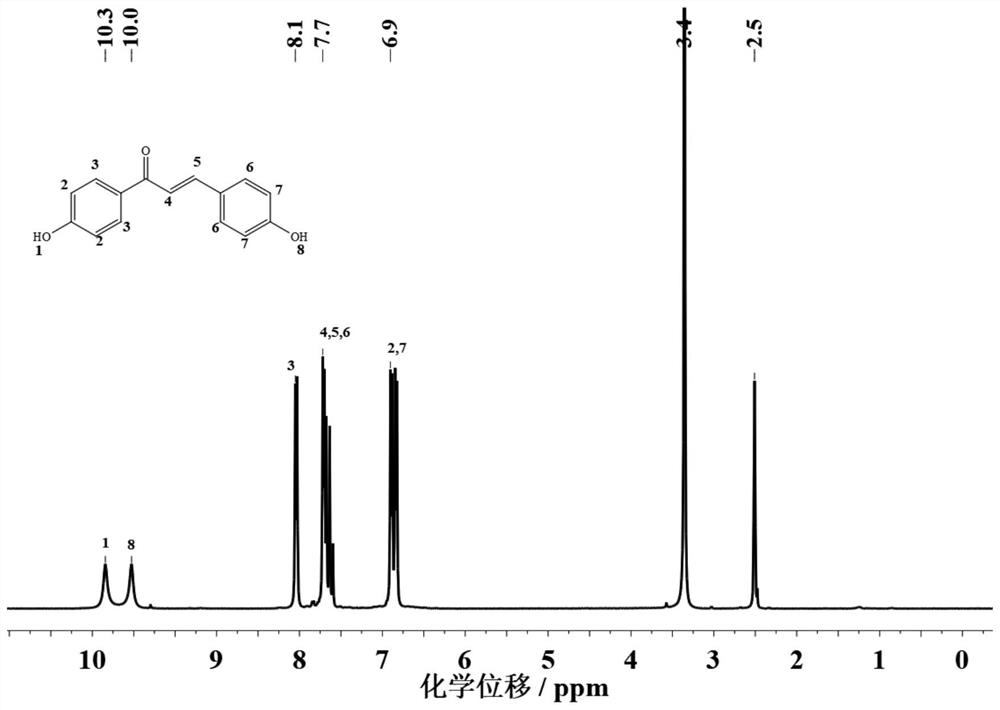
[0030] 1H NMR (400Hz, DMSO-d6): 10.0 (s, 1H), 10.3 (s, 1H), 8.1 (d, 2H), 7.64-7.72 (m, 4H), 6.8-6.9 (m, 4H).
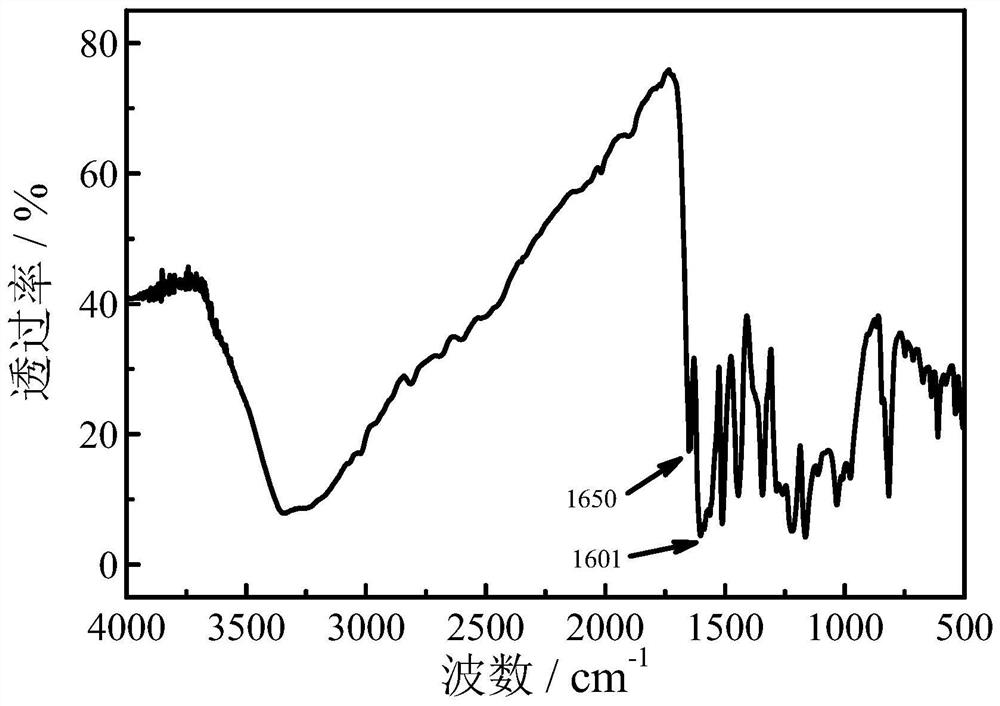
[0031] FTIR: 1650cm -1 It is -C=O stretching vibration absorption peak, 1601cm -1 It is -C=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com