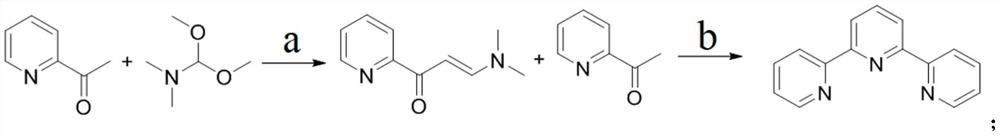
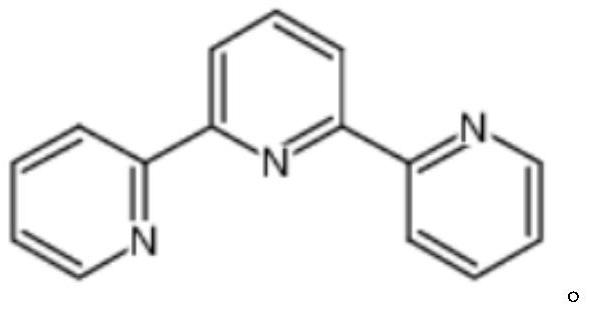
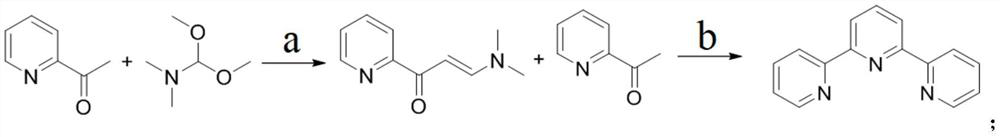
Method for large-scale production of alpha, alpha, alpha-terpyridyl
A terpyridine and pyridyl-based technology, applied in the direction of organic chemistry, can solve the problems of high toxicity, cumbersome post-treatment, and a yield of only 15%, achieving mild reaction conditions, significant economic value, and easy purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Preparation of intermediate 1-(3-pyridyl)-3-(dimethylamino)-2-propen-1-one
[0032] At room temperature, add 94L of tetrahydrofuran to the high and low temperature jacketed reaction kettle, then add 2-acetylpyridine (9.4kg, 1.0eq), N,N-dimethylformamide dimethyl acetal (11.2kg, 1.20 eq) and amidinothiourea (920g, 0.10eq), be warming up to reflux reaction, monitor reaction process by TLC, when reaction finishes, after making reaction solution be cooled to room temperature, pour reaction solution into 20L concentration and be 40% hydrogen oxidation solution sodium aqueous solution, then extracted twice with 50L dichloromethane, combined the organic phases, washed the obtained organic phases with saturated brine, dried over anhydrous sodium sulfate, and concentrated, and the obtained crude product was recrystallized with petroleum ether to obtain 5.77 kg intermediate Entity: 1-(3-pyridyl)-3-(dimethylamino)-2-propen-1-one, the molar yield is 42.0%.
Embodiment 2
[0033] Embodiment 2: Preparation of intermediate 1-(3-pyridyl)-3-(dimethylamino)-2-propene-1-one
[0034] At room temperature, add 94L of tetrahydrofuran to the high and low temperature jacketed reaction kettle, then add 2-acetylpyridine (9.4kg, 1.0eq), N,N-dimethylformamide dimethyl acetal (11.2kg, 1.20 eq) and tetramethylguanidine (900g, 0.10eq), be warming up to reflux reaction, monitor reaction process by TLC, when reaction finishes, after making reaction solution be cooled to room temperature, pour reaction solution into 20L concentration and be 40% hydroxide sodium aqueous solution, and then extracted twice with 50L dichloromethane, combined the organic phases, washed the obtained organic phases with saturated brine, dried over anhydrous sodium sulfate, and concentrated, and the obtained crude product was recrystallized with petroleum ether to obtain 6.65 kg intermediate Entity: 1-(3-pyridyl)-3-(dimethylamino)-2-propen-1-one, the molar yield is 48.4%.
Embodiment 3
[0035] Embodiment 3: Preparation of intermediate 1-(3-pyridyl)-3-(dimethylamino)-2-propen-1-one
[0036] At room temperature, add 94L of tetrahydrofuran to the high and low temperature jacketed reaction kettle, then add 2-acetylpyridine (9.4kg, 1.0eq), N,N-dimethylformamide dimethyl acetal (11.2kg, 1.20 eq) and 3-guanidino-L-alanine hydrochloride (1.42kg, 0.10eq), be warming up to reflux reaction, monitor reaction process by TLC, when reaction finishes, after making reaction solution cool to room temperature, reaction solution Pour into 20L of 40% aqueous sodium hydroxide solution, then extract twice with 50L of dichloromethane, combine the organic phases, wash the gained organic phase with saturated brine, dry over anhydrous sodium sulfate, and concentrate. Petroleum ether was recrystallized to obtain 4.65 kg of an intermediate: 1-(3-pyridyl)-3-(dimethylamino)-2-propen-1-one, with a molar yield of 33.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com