1,2-diaryl-2-propenyl-1-ketone compounds and use thereof
A ketone compound, disubstituted technology, applied in the field of medicine, can solve the problem that the research on anti-tumor activity has not yet been reported, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
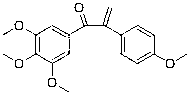
[0095] Example 1: Preparation of 1-(3,4,5-trimethoxyphenyl)-2-(4-methoxyphenyl)-2-propen-1-one (compound 1)
[0096] 1-(3,4,5-trimethoxyphenyl)-2-(4-methoxyphenyl)ethanone (0.1 g, 0.316 mmol) was dissolved in methanol, and 0.1 equivalent of piperidine ( 2.69 mg, 0.0316 mmol), 0.16 equivalents of glacial acetic acid (3.062 mg, 0.051 mmol) and 4.2 equivalents of paraformaldehyde (39.8 mg, 1.327 mmol), react at 50°C for 2 hours. After the reaction was completed, excess paraformaldehyde in the reaction solution was filtered off, the solvent was evaporated to dryness under reduced pressure, and then extracted with ethyl acetate. The organic layer was washed with saturated sodium chloride solution and dried over anhydrous sodium sulfate. The solvent was evaporated by a rotary evaporator, and the product was obtained by separation and purification by column chromatography. Yield 92.0%; The structural formula of compound 1, 1 H-NMR and MS data are listed in Table-1 below.
Embodiment 2
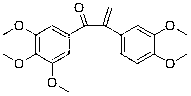
[0097] Example 2: Preparation of 1-(3,4,5-trimethoxyphenyl)-2-(3,4-dimethoxyphenyl)-2-propen-1-one (compound 2)
[0098] Except using corresponding raw materials, compound 2 is prepared in the same manner as in Example 1, and the yield is 85.2%; the structural formula of compound 2, 1 H-NMR and MS data are listed in Table-1 below.
Embodiment 3
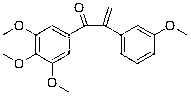
[0099] Example 3: Preparation of 1-(3,4,5-trimethoxyphenyl)-2-(3-methoxyphenyl)-2-propen-1-one (compound 3)
[0100] Except using corresponding raw materials, compound 3 is prepared in the same manner as in Example 1, and the yield is 88.5%; the structural formula of compound 3, 1 H-NMR and MS data are listed in Table-1 below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com