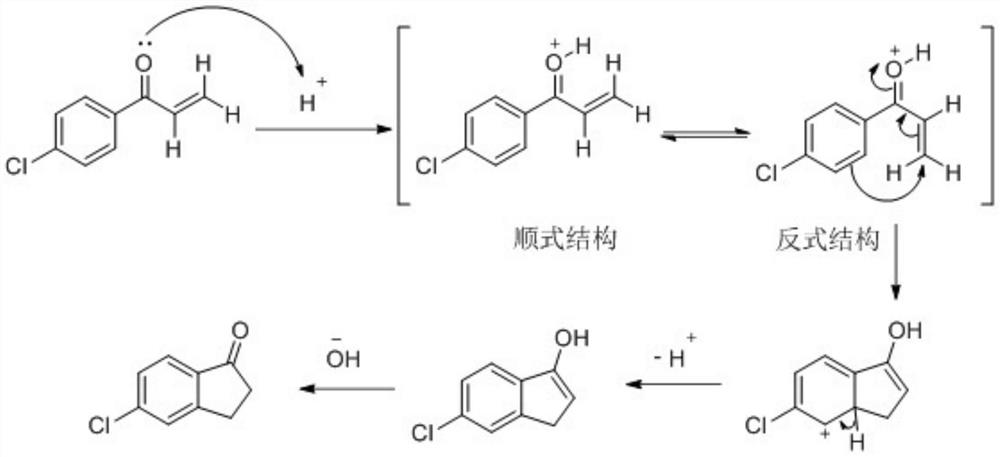
Synthesis method of 5-chloro-1-indanone
A synthesis method and indanone technology are applied in the field of synthesis of 5-chloro-1-indanone, which can solve the problems of potential safety hazards, high temperature, carbonization, etc., so as to improve environmental protection benefits, reduce the generation of tar, and improve product quality. and yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Weigh 175.2 g (1.0 mol) of 1-(4-chlorophenyl)-2-propen-1-one with a purity of 95% and 350 g of toluene, place in a high-pressure reaction flask, and stir to dissolve.
[0027] (2) Place the reaction bottle in a low-temperature tank and stir to lower the temperature. When the temperature in the reaction bottle drops to -5~0°C, start to pass hydrogen chloride gas slowly. React with hydrogen chloride.
[0028] (3) After 3 hours of aeration reaction, the temperature of the system no longer showed an upward trend, and 15.0% of 1-(4-chlorophenyl)-2-propen-1-one was detected by sampling. Close the tail gas venting valve of the reaction bottle, continue to pass hydrogen chloride gas, close the hydrogen chloride venting valve when the pressure of the high-pressure reaction bottle reaches 0.2-0.25 MPa, and continue the heat preservation reaction at 0-5 °C for 2 hours.
[0029] (4) Sampling and detection of 1-(4-chlorophenyl)-2-propen-1-one remaining at 0.2%, the reaction is ...
Embodiment 2
[0031] (1) Weigh 175.2 g (1.0 mol) of 1-(4-chlorophenyl)-2-propen-1-one with a purity of 95% and 400 g of toluene, place in a high-pressure reaction flask, and stir to dissolve.
[0032] (2) Place the reaction bottle in a low-temperature tank and stir to lower the temperature. When the temperature in the reaction bottle drops to -5-0°C, start to pass hydrogen chloride gas slowly. React with hydrogen chloride.
[0033] (3) After 2 hours of aeration reaction, the temperature of the system no longer showed an upward trend, and 15.4% of 1-(4-chlorophenyl)-2-propen-1-one was detected by sampling. Close the tail gas venting valve of the reaction bottle, continue to pass hydrogen chloride gas, close the hydrogen chloride venting valve when the pressure of the high-pressure reaction bottle reaches 0.2-0.25 MPa, and continue the heat preservation reaction at 0-5 °C for 2 hours.
[0034] (4) Sampling and detection of 1-(4-chlorophenyl)-2-propen-1-one remaining at 0.3%, the reaction is ...
Embodiment 3
[0036] (1) Weigh 175.2 g (1.0 mol) of 1-(4-chlorophenyl)-2-propen-1-one with a purity of 95% and 400 g of methanol, place in a high-pressure reaction flask, and stir to dissolve.
[0037] (2) Place the reaction bottle in a low-temperature tank and stir to lower the temperature. When the temperature in the reaction bottle drops to -5~0°C, start to pass hydrogen chloride gas slowly. React with hydrogen chloride.
[0038] (3) After 2 hours of ventilation reaction, the temperature of the system no longer showed an upward trend, and 13.0% of 1-(4-chlorophenyl)-2-propen-1-one was detected by sampling. Close the tail gas venting valve of the reaction bottle, continue to pass hydrogen chloride gas, close the hydrogen chloride venting valve when the pressure of the high-pressure reaction bottle reaches 0.2-0.25 MPa, and continue the heat preservation reaction at 0-5 °C for 2 hours.
[0039] (4) Sampling and detection of 1-(4-chlorophenyl)-2-propen-1-one remaining at 0.28%, the reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com