Method for desulfurization cogenerating of hydrogen and sulfuric acid
A sulfuric acid and hydrogen technology, applied in chemical instruments and methods, separation methods, sulfur compounds, etc., can solve the problems of electrode deactivation, difficult operation, large loss of iodine, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
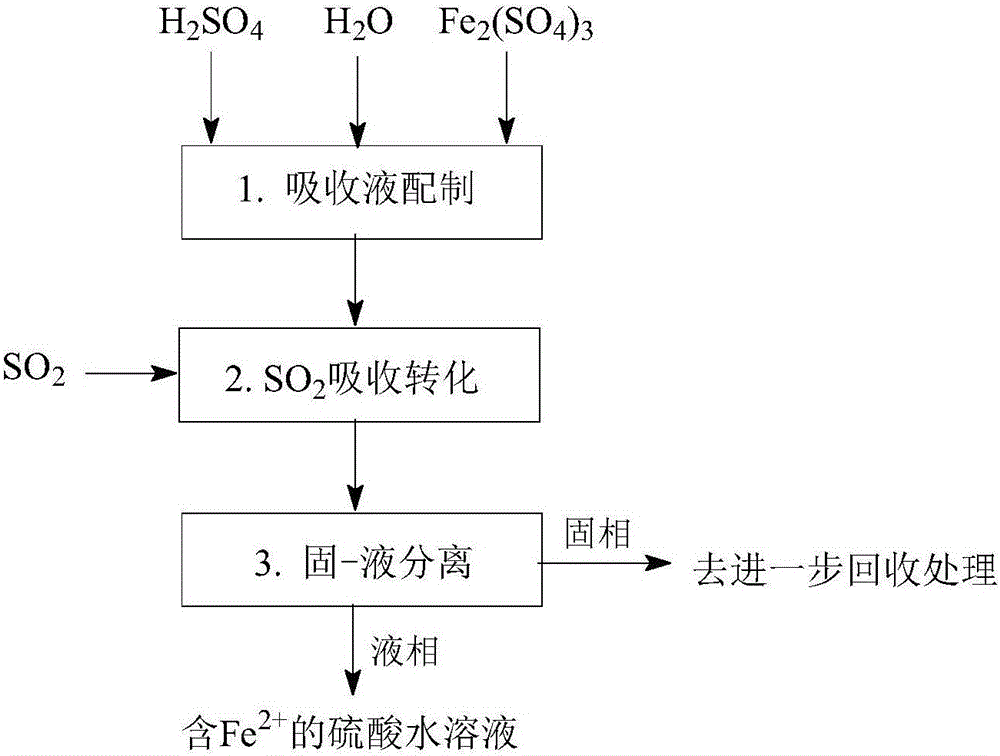
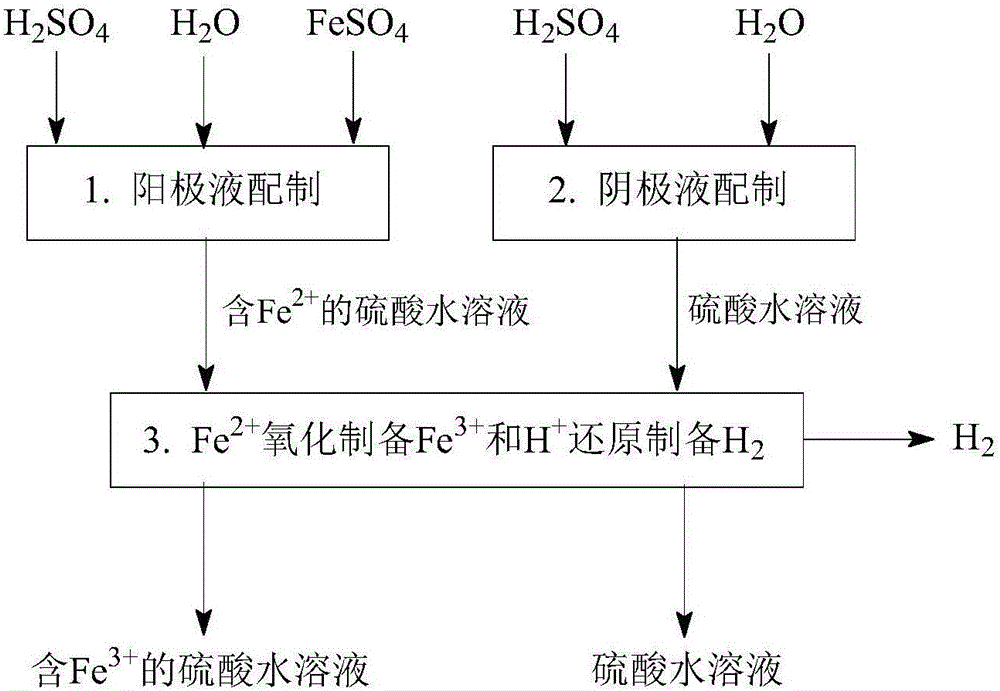
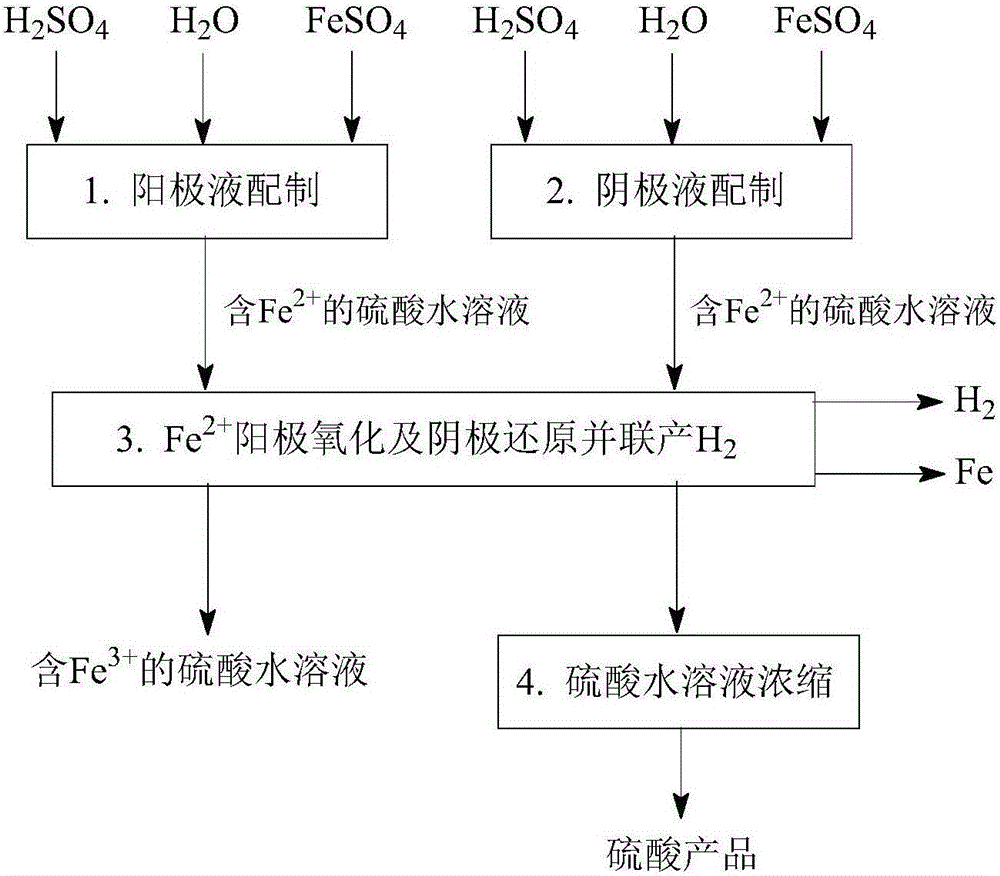
[0161] Such as Figure 1-Figure 5 As shown, a method for desulfurization and co-production of hydrogen and sulfuric acid, including "SO 2 Absorption and transformation", "Fe 3+ Regeneration and Cogeneration H 2 "," Fe 2+ Removal and co-production of H 2 "Three main steps:
[0162] Specific steps are as follows:
[0163] (1) SO 2 absorption transformation process
[0164] Such as figure 1 As shown, a SO 2 Absorption conversion process, especially with Fe-containing 3+ Aqueous sulfuric acid solution as absorbent, Fe 3+ As an oxidizing agent, the SO 2 Absorb dissolved in sulfuric acid aqueous solution, absorb dissolved SO 2 with Fe 3+ The reaction converts to H 2 SO 4 , while Fe 3+ is reduced to Fe 2+ ; The obtained Fe 2+ The sulfuric acid aqueous solution can further Fe 2+ Oxidation regeneration to Fe 3+ Afterwards, it can be recycled as an absorbent, and it can also be further removed by Fe 2+ And concentration operation to prepare sulfuric acid products. ...
Embodiment 2
[0180] A method for desulfurization and co-production of hydrogen and sulfuric acid, characterized in that the method includes "SO 2 Absorption and transformation", "Fe 3+ Regeneration and Cogeneration H 2 "," Fe 2+ Removal and co-production of H 2 "Three main steps:
[0181] Specific steps are as follows:
[0182] (1) SO 2 absorption transformation process
[0183] Such as figure 1 As shown, a SO 2 Absorption conversion process, especially with Fe-containing 3+ Aqueous sulfuric acid solution as absorbent, Fe 3+ As an oxidizing agent, the SO 2 Absorb dissolved in sulfuric acid aqueous solution, absorb dissolved SO 2 with Fe 3+ The reaction converts to H 2 SO 4 , while Fe 3+ is reduced to Fe 2+ ; The obtained Fe 2+ The sulfuric acid aqueous solution can further Fe 2+ Oxidation regeneration to Fe 3+ Afterwards, it can be recycled as an absorbent, and it can also be further removed by Fe 2+ And concentration operation to prepare sulfuric acid products. Specif...
Embodiment 3
[0199] A method for desulfurization and co-production of hydrogen and sulfuric acid, including "SO 2 Absorption and transformation", "Fe 3+ Regeneration and Cogeneration H 2 "," Fe 2+ Removal and co-production of H 2 "Three main steps: The specific steps are as follows:
[0200] (1) SO 2 absorption transformation process
[0201] Such as figure 1 As shown, a SO 2 Absorption conversion process, especially with Fe-containing 3+ Aqueous sulfuric acid solution as absorbent, Fe 3+ As an oxidizing agent, the SO 2 Absorb dissolved in sulfuric acid aqueous solution, absorb dissolved SO 2 with Fe 3+ The reaction converts to H 2 SO 4 , while Fe 3+ is reduced to Fe 2+ ; The obtained Fe 2+ The sulfuric acid aqueous solution can further Fe 2+ Oxidation regeneration to Fe 3+ Afterwards, it can be recycled as an absorbent, and it can also be further removed by Fe 2+ And concentration operation to prepare sulfuric acid products. Specific steps are as follows:
[0202] (1)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com