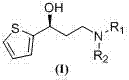
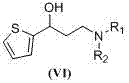
Preparation method of (S)-3-N,N-disubstituted amino-1-(2-thienyl)-1-propanol
A two-substituted, thienyl-based technology is applied in the field of preparation of duloxetine intermediates, which can solve the problems of high environmental pressure, large amount of paraformaldehyde and dimethylamine hydrochloride, and inability to recycle and reuse, and achieves less three wastes. , the effect of low cost and high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: N,N -Synthesis of dimethylamino-1-(2-thienyl)-1-propenone
[0043] 2-acetylthiophene:bis(trichloromethyl)carbonate according to the molar ratio of substances: N,N -Disubstituted formamide: 1.0:0.32:1.15:1.5 feed of organic base; 12.6g of 2-acetylthiophene, 9.5g of bis(trichloromethyl)carbonate, N,N - Disubstituted carboxamides are N, N -Dimethylformamide, the feeding quality is 8.4g, the organic base is barium hydroxide, and the feeding quality is 17.1g; Organic solvent A is methanol 125mL, and its volume consumption is 10mL / g of 2-acetylthiophene quality; Solvent B is For 10 mL of dichloromethane, the volume used is 1 mL / g of the mass of bis(trichloromethyl)carbonate.
[0044] First put N,N - Dimethylformamide was added to the reaction flask, mechanical stirring was started, the temperature was cooled to 0°C in an ice bath, and a dichloromethane solution in which bis(trichloromethyl)carbonate was dissolved was added dropwise. After the dropwise additio...
Embodiment 2
[0046] 2-acetylthiophene:bis(trichloromethyl)carbonate according to the molar ratio of substances: N,N -Disubstituted formamide: 1.0:0.7:2.5:2 feed of organic base; 12.6g of 2-acetylthiophene, 20.7g of bis(trichloromethyl)carbonate, N,N - Disubstituted carboxamides are N,N -Dimethylformamide, the feeding quality is 18.3g, the organic base is sodium methylate, and the feeding quality is 10.8g; Organic solvent A is methanol 250mL, and its volumetric dosage is 20mL / g of 2-acetylthiophene quality; Solvent B is 1 , 60mL of 4-dioxane, the volume dosage is 3mL / g of the mass of bis(trichloromethyl)carbonate.
[0047] 80 ℃ of temperature of reaction, other operations are with embodiment 1, obtain N,N - 16.7 g of dimethylamino-1-(2-thienyl)-1-propenone, yield 92.2%.
Embodiment 3
[0049] 2-acetylthiophene:bis(trichloromethyl)carbonate according to the molar ratio of substances: N,N -Disubstituted formamide: 1.0:1.0:3.6:3 feed of organic base; 12.6g of 2-acetylthiophene, 29.7g of bis(trichloromethyl)carbonate, N,N - Disubstituted carboxamides are N,N -Dimethylformamide, the feeding quality is 26.3g, the organic base is sodium acetate, and the feeding quality is 41g; Organic solvent A is methanol 380mL, and its volumetric dosage is 30mL / g of 2-acetylthiophene quality; Solvent B is ethyl acetate Esters 150mL, the volume dosage is 5mL / g of bis(trichloromethyl)carbonate mass.
[0050] 60 DEG C of temperature of reaction, other operation is with embodiment 1, obtains N,N - 15.7 g of dimethylamino-1-(2-thienyl)-1-propenone, yield 86.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com