Application of silylamino rare earth compound in catalysis of reaction of isatin compound and cyclopropenone compound
A technology of rare earth compounds and cyclopropenone, applied in organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, chemical instruments and methods, etc. The steps are cumbersome and other problems, to achieve the effect of short reaction time, low reaction cost, and simple and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
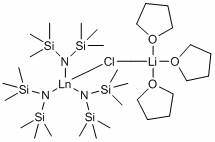
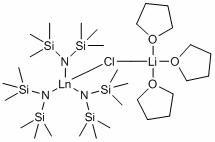
[0035] Embodiment one: catalyst [(Me 3 Si) 2 N] 3 La( m -Cl)Li(THF) 3 Synthesis
[0036] At -10°C, the n -BuLi in hexane solution (60 mmol, 2.52 M) was added dropwise to the (Me 3 Si) 2 NH (60mmol) in a 100 mL Schlenk reaction flask and reacted at room temperature for 30 minutes. The above reaction solution was added to anhydrous LaCl 3 (20 mmol) in THF (30 mL) and stirred overnight at room temperature. The solvent was removed under reduced pressure, and the obtained solid powder was extracted with hot toluene to remove LiCl, concentrated, placed at 0°C, and a large number of crystals were precipitated, which was the desired lanthanum siliconamide compound, with a yield of 85%.
[0037] Other catalysts can refer to the preparation method of Example 1.
Embodiment 2
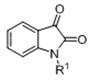
[0038] Embodiment two: [(Me 3 Si) 2 N] 3 Yb( m -Cl)Li(THF) 3 catalytic N Preparation of pyrano[2,3- b ]indol-2-one compounds
[0039] In the reaction flask treated with dehydration and deoxygenation, weigh [(Me 3 Si) 2 N] 3 Yb( m -Cl)Li(THF) 3 (43.8 mg, 0.048 mmol), diethyl phosphite (37 μL, 0.29 mmol), N-Ethyl isatin (50.7 mg, 0.29 mmol), mixed with conventional stirring for 30 minutes, then added solvent (1.0 mL), 2,3-diphenylcyclopropenone (50 mg, 0.24 mmol), stirred at 50 °C After 1.5 hours, add water to terminate the reaction, extract three times with ethyl acetate, dry the extract with anhydrous sodium sulfate, filter, remove the solvent under reduced pressure, and finally perform flash column chromatography on a silica gel column (eluent: ethyl acetate:petroleum ether=1 : 10) Obtain a yellow solid product; the theoretical molecular formula and main NMR test data of the obtained product are as follows, and it can be seen from the analysis that the actual synt...
Embodiment 3
[0045] Embodiment three: [(Me 3 Si) 2 N] 3 La( m -Cl)Li(THF) 3 catalytic N Preparation of pyrano[2,3- b ]indol-2-one compounds
[0046] In the reaction flask treated with dehydration and deoxygenation, weigh [(Me 3 Si) 2 N] 3 La( m -Cl)Li(THF) 3 (42.2 mg, 0.048 mmol), diethyl phosphite (37 μL, 0.29 mmol), N -Ethyl isatin (50.7 mg, 0.29 mmol), mixed with conventional stirring for 30 minutes, then added toluene (1.0 mL), 2,3-diphenylcyclopropenone (50 mg, 0.24 mmol), stirred at 50°C After 1.5 hours, add water to terminate the reaction, extract three times with ethyl acetate, dry the extract with anhydrous sodium sulfate, filter, remove the solvent under reduced pressure, and finally perform flash column chromatography on a silica gel column (eluent: ethyl acetate:petroleum ether=1 :10) to obtain a yellow solid product with a yield of 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com