Gamma-alkenyl substituted butenolide or butenolactam compound and asymmetric synthesis method and ligand of gamma-alkenyl substituted butenolide or butenolactam compound
A technology of butenolactam and butenolactone, applied in asymmetric synthesis, organic compound/hydride/coordination complex catalyst, organic chemical method, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
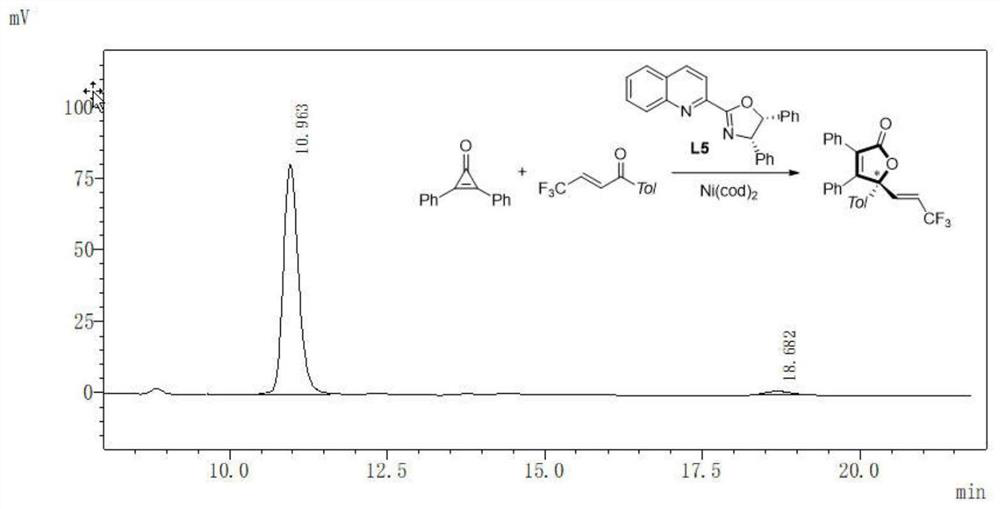
[0026] Aiming at the deficiencies of the prior art, the object of the present invention is to provide a γ-alkenyl substituted butenolactone or butenolactam compound and an asymmetric synthesis method thereof.
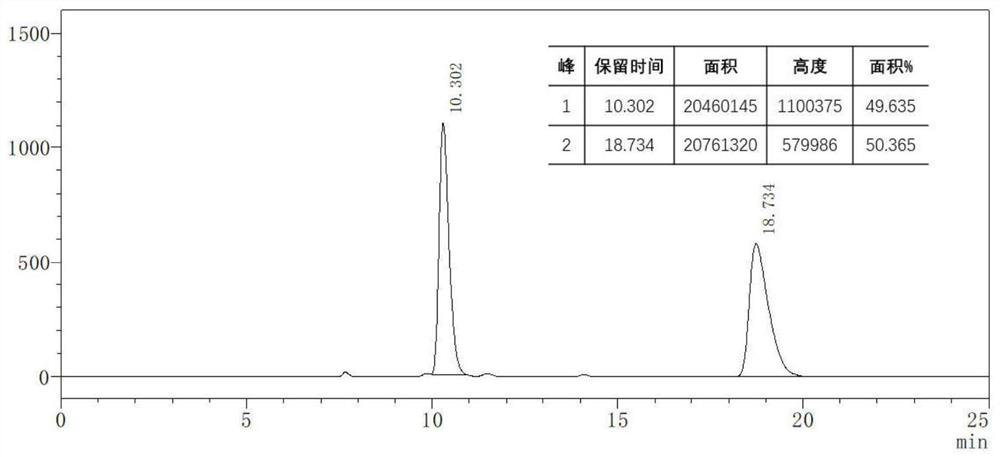
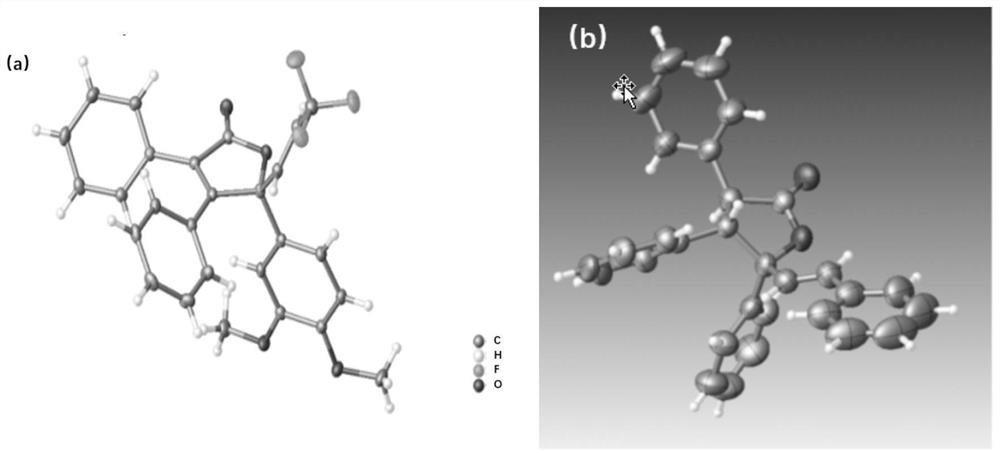
[0027] The method of the present invention utilizes Ni(0)-catalyzed intermolecular C-C bond activation and selective C=O insertion reaction to realize the asymmetric [3+2] cycloaddition reaction of cyclopropanone derivatives and unsaturated ketone derivatives, and A new type of γ-alkenyl-substituted butenolactone or butenolactam compound was prepared, which has the advantages of cheap and easy-to-obtain catalyst, controllable chirality of the product, simple reaction, mild conditions, high yield and enantioselectivity, etc. .
[0028] In the present invention, the challenge of realizing the enantioselective cycloaddition reaction between cycloacetone derivatives and unsaturated ketone derivatives is that (a) cycloacetone derivatives are easily dimerized into spironolact...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com