Method for synthesizing chalcone derivative with anti-malaria activity
A technology of chalcone derivatives and synthetic methods, which is applied in the field of drug synthesis, can solve the serious and fatal problems of falciparum malaria, and achieve the effect of short production cycle and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
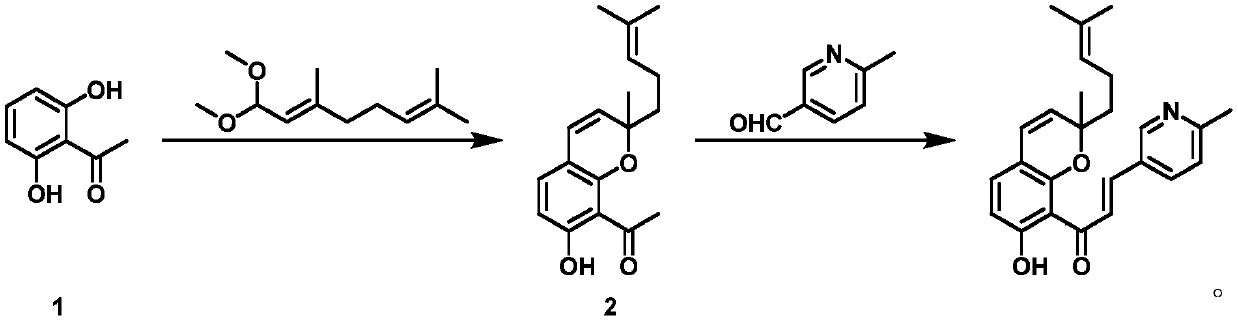
[0029] Synthesis conditions of 1-(7-hydroxy-2-methyl-2-(4-methylpent-3-en-1-yl)-2H-benzopyran-8-yl)ethan-1-one 2 for:
[0030] Mix 2,6-dihydroxyacetophenone 1 (72g), citral dimethyl acetal (141g) and pyridine (370g) and stir at 100-150°C for 10-15 hours; add ethyl acetate to the system (800mL) and saturated copper sulfate solution (600mL), stirred for 1-2 hours, and the organic phase was separated; n-heptane (1000mL) was added to the organic phase, stirred for 2-5 hours, filtered to obtain 1-(7-hydroxy- 2-Methyl-2-(4-methylpent-3-en-1-yl)-2H-benzopyran-8-yl)ethan-1-one 2 (83 g, 61%).
[0031] 1 H NMR (400MHz, DMSO-d6)δ=16.5(s,1H),7.93(d,1H),6.88(d,1H),6.44(d,1H),5.92(d,1H),5.20(t, 1H),2.54(s,3H),1.94(m,3H),1.82(m,3H),1.70(s,3H),1.62(s,2H),1.24(s,3H)ppm; m / z( MS-ESI):287.35[M+1] + .
[0032](E)-1-(7-Hydroxy-2-methyl-2-(4-methylpent-3-en-1-yl)-2H-benzopyran-8-yl)-3-(6 -methylpyridin-3-yl) prop-2-en-1-one synthesis conditions are:
[0033] 1-(7-Hydroxy-2-methyl-2-(4-methy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com