Capsaicin receptor antagonist for treating chronic inflammatory pain
A capsaicin receptor and antagonist technology, applied in the field of drug synthesis, can solve problems such as not entering clinical trials, and achieve the effects of short production cycle and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
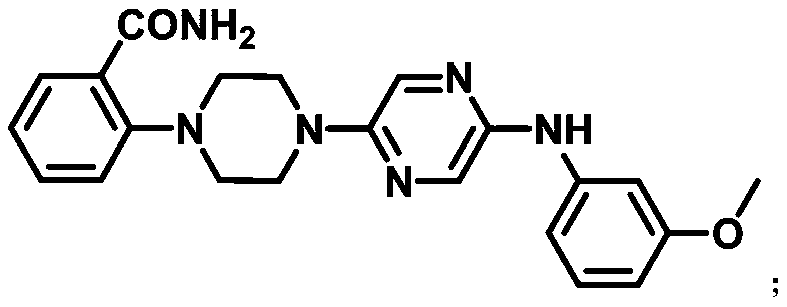
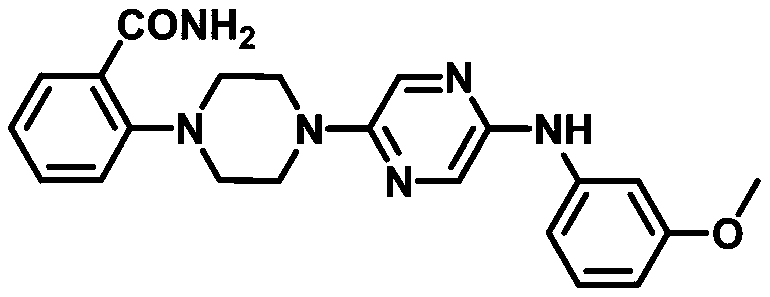
[0034] A compound 2-(4-(5-((3-methoxyphenyl)amino)pyrazin-2-yl)piperazin-1-yl)benzamide can be used as capsaicin receptor antagonist For the treatment of chronic inflammatory pain, its structural formula is as follows:
[0035]
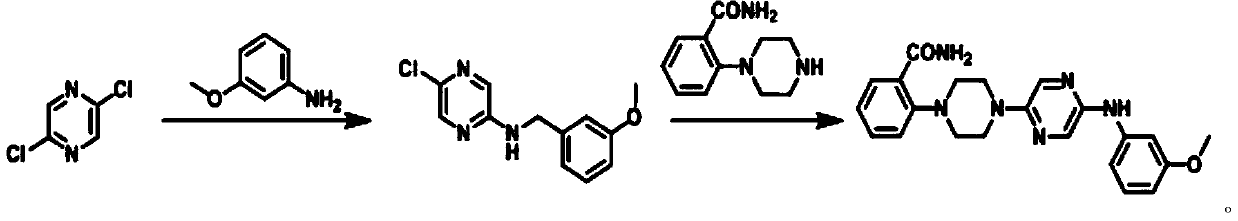
[0036] The synthetic method of capsaicin receptor antagonist 2-(4-(5-((3-methoxyphenyl) amino) pyrazin-2-yl) piperazin-1-yl) benzamide is:
[0037]
[0038] Wherein, the synthesis condition of 5-chloro-N-(3-methoxyphenyl)pyrazin-2-amine is:
[0039] Mix 2,5-dichloropyrazine (58.5g), 3-methoxyaniline (53.2g), potassium tert-butoxide (70.6g) and dimethylformamide (300mL), heat to 40°C, Stir for 2 hours; cool down to 15°C, add methyl tert-butyl ether (600mL) to the system, stir for 2 hours, and filter to obtain 5-chloro-N-(3-methoxyphenyl)pyrazine-2 - Amine (72.7 g, 74%).
[0040] 1 H NMR (CDCl 3 ,400MHz): δ(ppm)=8.22(s,1H),8.16(s,1H),7.31(t,1H),7.12-6.94(m,3H),5.29(brs,1H),4.44(s, 2H), 3.78(s, 3H); 13 C NMR (CDCl 3 ,100MHz): δ(ppm)=160.3,1...
Embodiment 2
[0045] A compound 2-(4-(5-((3-methoxyphenyl)amino)pyrazin-2-yl)piperazin-1-yl)benzamide can be used as capsaicin receptor antagonist For the treatment of chronic inflammatory pain, its structural formula is as follows:
[0046]
[0047] The synthetic method of capsaicin receptor antagonist 2-(4-(5-((3-methoxyphenyl) amino) pyrazin-2-yl) piperazin-1-yl) benzamide is:
[0048]
[0049] Wherein, the synthesis condition of 5-chloro-N-(3-methoxyphenyl)pyrazin-2-amine is:
[0050] Mix 2,5-dichloropyrazine (58.5g), 3-methoxyaniline (106.4g), diisopropylethylamine (141.2g) and N-methylpyrrolidone (600mL) and heat to 50 ℃, stirred for 3 hours; cooled to 20 ℃, added petroleum ether (900mL) to the system, stirred for 3 hours, filtered to obtain 5-chloro-N-(3-methoxyphenyl)pyrazine-2- Amine (80 g, 70%).
[0051] 1 H NMR (CDCl 3 ,400MHz): δ(ppm)=8.22(s,1H),8.16(s,1H),7.31(t,1H),7.12-6.94(m,3H),5.29(brs,1H),4.44(s, 2H), 3.78(s, 3H); 13 C NMR (CDCl 3 ,100MHz): δ(ppm)=160.3,155....
Embodiment 3
[0056] A compound 2-(4-(5-((3-methoxyphenyl)amino)pyrazin-2-yl)piperazin-1-yl)benzamide can be used as capsaicin receptor antagonist For the treatment of chronic inflammatory pain, its structural formula is as follows:
[0057]
[0058] The synthetic method of capsaicin receptor antagonist 2-(4-(5-((3-methoxyphenyl) amino) pyrazin-2-yl) piperazin-1-yl) benzamide is:
[0059]
[0060] Wherein, the synthesis condition of 5-chloro-N-(3-methoxyphenyl)pyrazin-2-amine is:
[0061] Mix 2,5-dichloropyrazine (58.5g), 3-methoxyaniline (159.6g), potassium hydroxide (211.8g), and dichloromethane (900mL), heat to 40-70°C, Stir for 4 hours; cool down to 22°C, add water (1200mL) to the system, stir for 4 hours, filter to obtain 5-chloro-N-(3-methoxyphenyl)pyrazin-2-amine (134g, 84%).
[0062] 1 H NMR (CDCl 3 ,400MHz): δ(ppm)=8.22(s,1H),8.16(s,1H),7.31(t,1H),7.12-6.94(m,3H),5.29(brs,1H),4.44(s, 2H), 3.78(s, 3H); 13 C NMR (CDCl 3 ,100MHz): δ(ppm)=160.3,155.8,144.7,141.2,139.4,136...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com