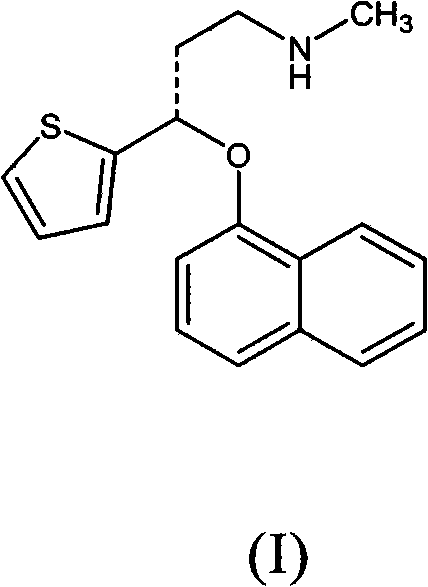
Delayed release pharmaceutical composition of duloxetine
A technology of duloxetine and composition, which is applied in the field of duloxetine sustained-release pharmaceutical composition, and can solve problems such as difficulties in enteric-coated preparations with drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
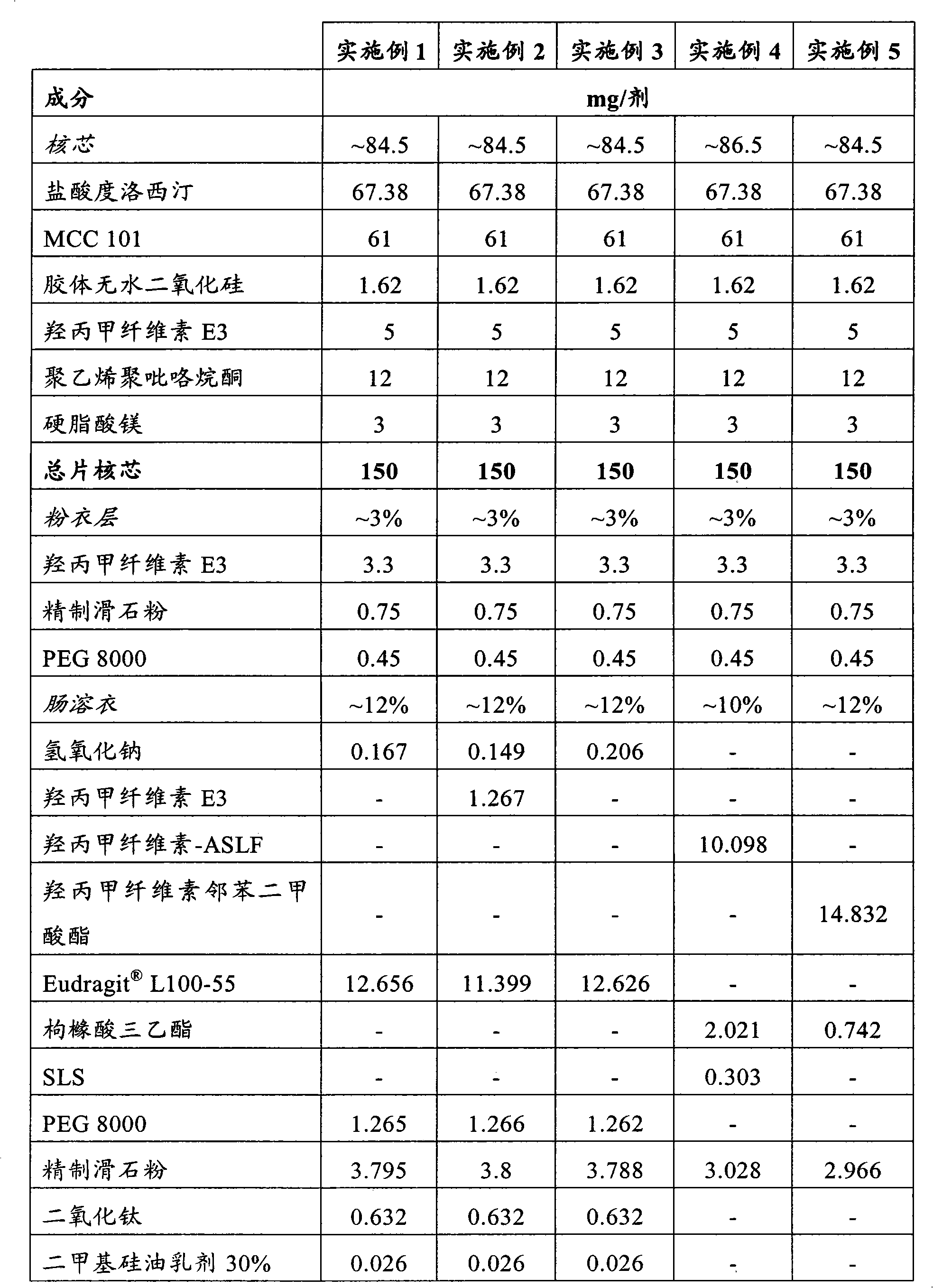
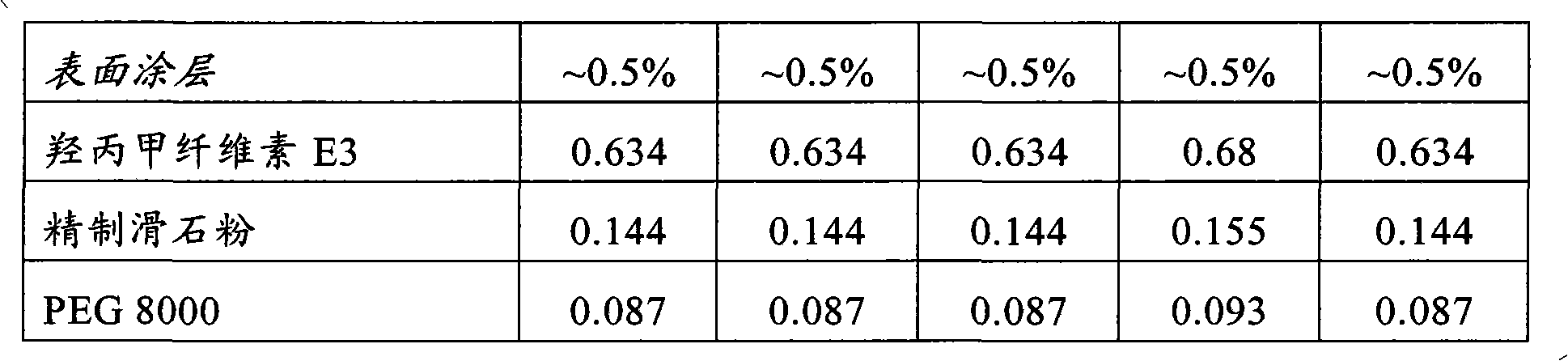
[0112] The following examples relate to hard gelatin capsules containing a tablet core composition prepared by standard wet granulation techniques. The capsule exemplifies a single 60 mg dose of duloxetine and contains 3 unit dosage forms in the form of tablets compressed at 50 mg each and each tablet contains 20 mg of duloxetine in hydrochloride form . The data in the following examples refer to the combined values of the three tablets in the capsule. It will of course be understood that the number of unit dosage forms and the actual effective amount in each unit dosage form may vary according to dosage requirements. For example a 20 mg dose of duloxetine may be formed in capsules, a single unit dosage form containing 20 mg duloxetine or an equivalent amount of a pharmaceutically acceptable salt, or two unit dosage forms each containing 10 mg duloxetine.
[0113]
[0114]
[0115]
[0116]
[0117] The following examples relate to duloxetine hydrochloride coat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com