Enteric coated table of duloxetine, and preparation method
A technology for enteric and coated tablets of duloxetine, which is applied in the field of enteric-coated oral coated tablets of duloxetine and the preparation thereof, can solve the problems that do not involve solid dispersion technology and solubilization methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
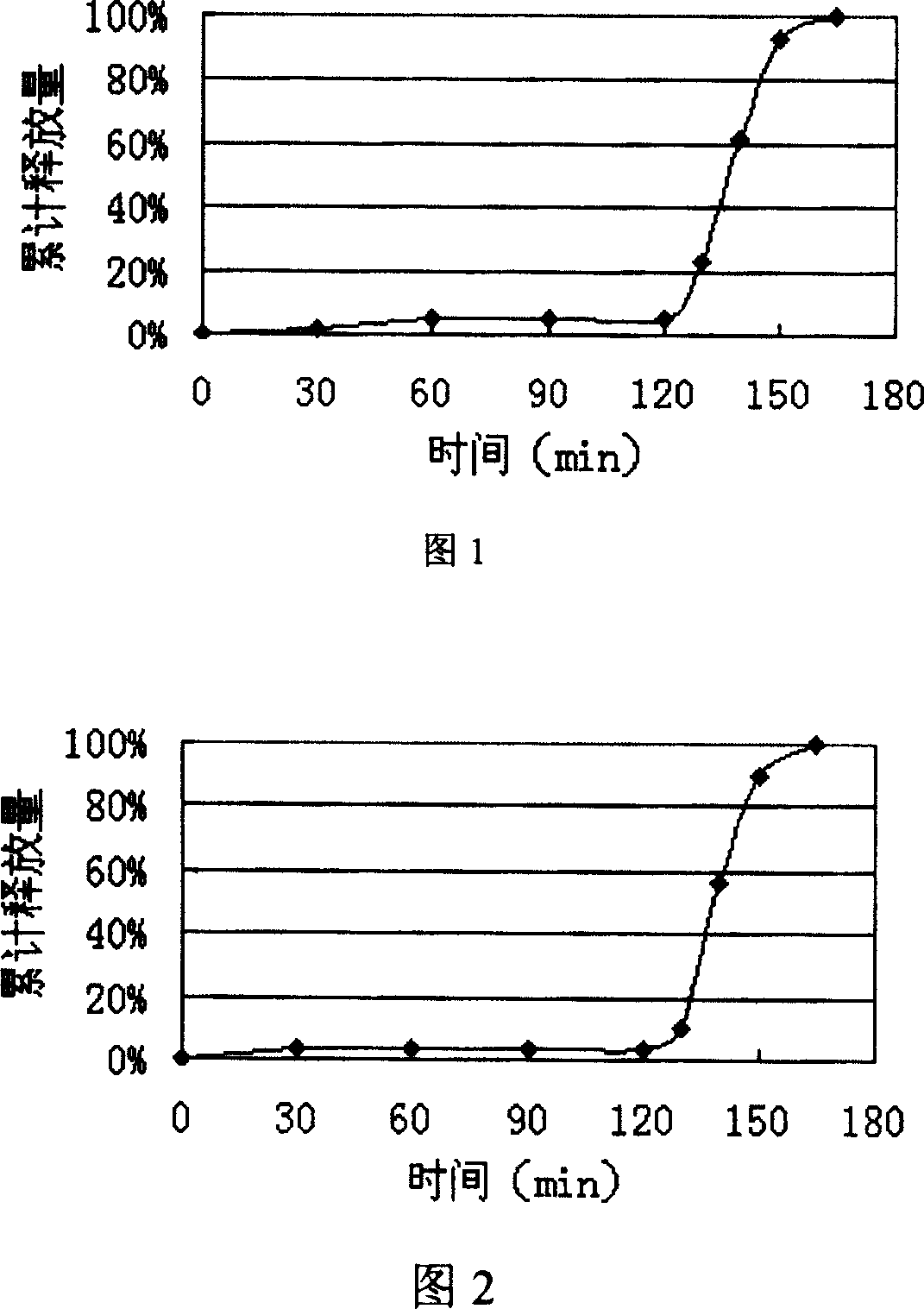
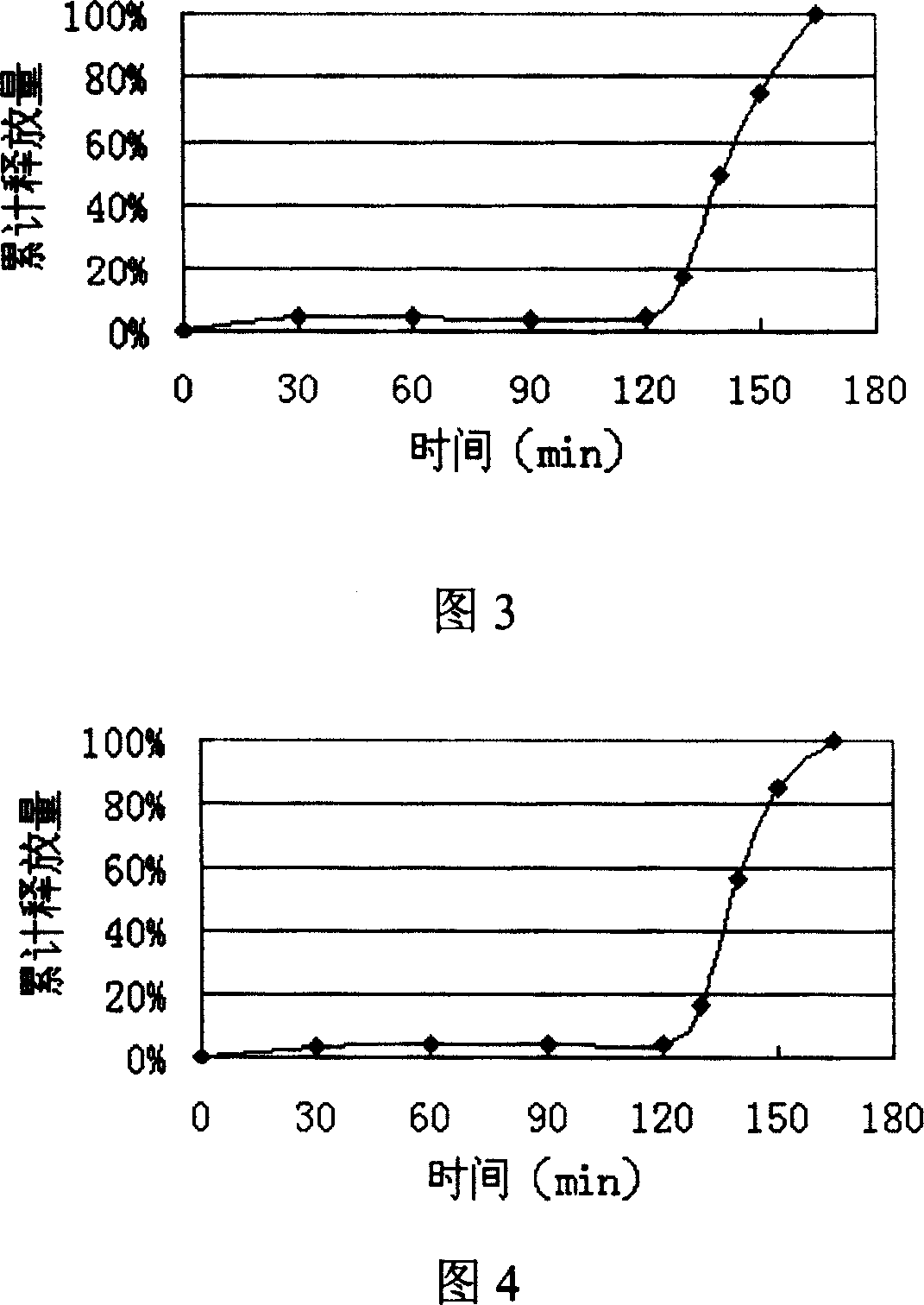
Image
Examples
Embodiment 1
[0055] Dissolve polyvinylpyrrolidone in an appropriate amount of ethanol solution, add the prescribed amount of duloxetine, add sucrose and lactose after it dissolves, stir and mix evenly, sieve, and dry in an oven to obtain duloxetine as a solid Dispersion, whole granules, mixed with prescribed amount of magnesium stearate and compressed into tablets to obtain plain tablet cores.
[0056] Preparation of the coating solution for the isolation layer: heat half of the prescribed amount of water to 70°C, pour in hypromellose, stir to disperse evenly and set aside. Add polyethylene glycol, talcum powder, and titanium dioxide to the other half of the water, use a high-shear homogenizer to homogenize for several minutes, and slowly pour the suspension into the hypromellose solution prepared in the previous step, slowly Stir at high speed for half an hour, and coat with a sugar pan under the continuous stirring of a magnetic stirrer.
[0057] Enteric-coating layer preparation...
Embodiment 2
0.63
[0062] Dissolve hypromellose in an appropriate amount of ethanol solution, add the prescribed amount of duloxetine, add sucrose and lactose after it dissolves, stir and mix evenly, sieve, and dry in an oven to obtain duloxetine The solid dispersion, the whole grain, mixed with the prescribed amount of micropowder silica gel, and then pressed into tablets to obtain plain tablet cores.
[0063] Preparation of the coating solution for the isolation layer: heat half of the prescribed amount of water to 70°C, pour in hypromellose, stir to disperse evenly and set aside. Add triethyl citrate, talc powder, and titanium dioxide to the other half of the water, homogenize with a high-shear homogenizer for several minutes, and slowly pour the suspension into the hypromellose solution prepared in the previous step , stirred at a slow speed for half an hour, and coated with a sugar pan under the continuous stirring of a magnetic stirrer.
[0064] Preparation of enteric laye...
Embodiment 3
[0068] Dissolve polyethylene glycol in an appropriate amount of ethanol solution, add the prescribed amount of duloxetine, add lactose and mannitol after it dissolves, stir and mix evenly, sieve, and dry in an oven to obtain duloxetine The solid dispersion, the whole grain, is mixed with the prescribed amount of talcum powder and compressed into tablets to obtain plain tablet cores.
[0069] Preparation of the coating solution for the isolation layer: dissolve polyvinylpyrrolidone in half of the prescribed amount of water. Add polyethylene glycol, talcum powder, and titanium dioxide to the other half of the water, and use a high-shear homogenizer to homogenize for several minutes. Slowly pour the suspension into the polyvinylpyrrolidone solution prepared in the previous step, and stir slowly For half an hour, coat with a icing pan under constant stirring with a magnetic stirrer.
[0070] Preparation of enteric layer coating solution: Weigh the required aqueous dispersi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com