Carbonyl reductase, gene thereof, and application thereof in preparing Duloxetine chiral intermediates
A technology of reductase and carbonyl, which is applied in the field of carbonyl reductase, its gene and the preparation of duloxetine chiral intermediates, which can solve the problems of difficult application of pharmaceutical intermediates, low optical purity, large waste discharge, etc. problems, to achieve the effect of large-scale industrial application prospects, simple conversion process, and stable coenzyme cycle system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
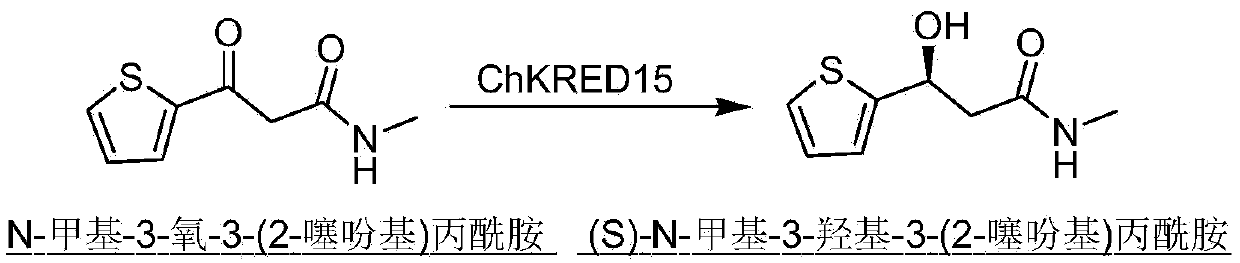
[0019] According to the whole genome sequencing information of Chryseobacterium sp.CA49, a large number of carbonyl reductases were excavated, one of which has the ability to catalyze the formation of (S)- The functional enzyme of N-methyl-3-hydroxy-3-(2-thienyl)propanamide is carbonyl reductase ChKRED15 involved in the present invention, and the gene acquisition process is a conventional operation method. Design the following primers: Forward: 5′-GCG GAATTC ATG AAA ACA GTA TTA ATT ACA GGC GCC-3′ (restriction site: EcoRI); reverse: 5′-GCG AAGCTT CTA CCA CGG ACT GAT TCC GG-3′ (restriction site: Hind III), using the genomic DNA of Chryseobacterium aureus CCTCC M2012484 as a template, the carbonyl reductase ChKRED15 gene was obtained by PCR amplification.
[0020] The PCR program was: pre-denaturation at 95°C for 5 min, denaturation at 94°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 1 min, 30 cycles, and finally extension at 72°C for 10 min.
[0021] After t...
Embodiment 2
[0024]Pick the single clone of the recombinant E.coli (pET28a-ChKRED15) constructed in Example 1, inoculate it in LB medium containing 50 μg / mL kanamycin, and culture it at 37°C and 180 rpm for 16 hours as a seed solution. % inoculum rate was transferred to fresh LB or TB medium (500mL capacity shake flask liquid 200mL medium), cultured with shaking at 37°C for 4h, and then added IPTG with a final concentration of 0.5mM to induce the expression of carbonyl reductase ChKRED15 gene. After induction at 30°C for 36h, cells were harvested by refrigerated centrifugation at 8000rpm for 10min at 4°C.
[0025] Bacteria can be used as biocatalysts or for protein purification.
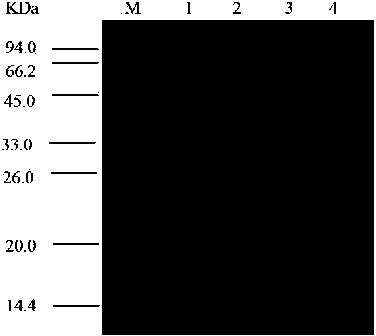
[0026] Example 3 Separation and purification of carbonyl reductase (ChKRED15)
Embodiment 3
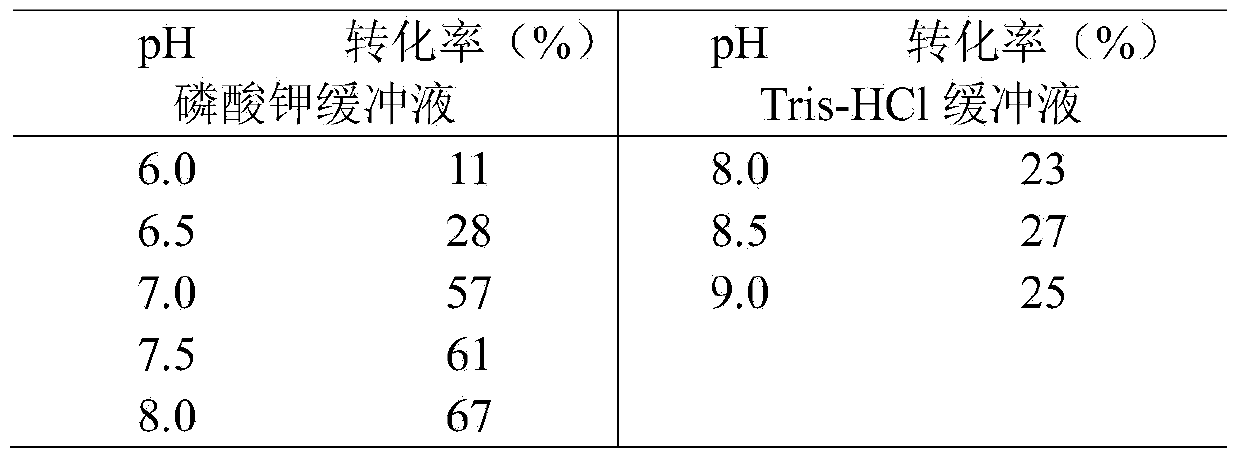
[0027] The bacteria obtained in Example 2 were resuspended in binding buffer (100mM, pH8.0 sodium phosphate buffer, containing 300mM NaCl, 5mM imidazole), crushed by a high-pressure homogenizer, centrifuged at 12000rpm for 15min, and the supernatant was mixed with the After incubation with the Ni affinity chromatography resin equilibrated with the above-mentioned binding solution, wash with washing buffer (100mM, pH8.0 sodium phosphate buffer, containing 300mM NaCl, 10mM imidazole) until there is basically no impurities, and then wash with elution buffer (100mM, pH8.0 sodium phosphate buffer, containing 300mM NaCl, 250mM imidazole) to elute and collect the target protein, after electrophoresis to identify the purity, combine the target protein and dialyze with dialysis buffer (100mM, pH8.0 potassium phosphate buffer) for 48h After ultrafiltration and concentration, the protein concentration was determined to be 8 mg / mL using the BCA Protein Assay kit, and the enzyme solution wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com