Method for asymmetric synthesis of duloxetine intermediate by carbonyl reductase
A carbonyl reductase, asymmetric technology, applied in the field of biocatalysis, can solve the problems of low level, low efficiency, and limited industrial application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Expression and purification of recombinant carbonyl reductase
[0050] LB medium (g / L): yeast extract 5.0, peptone 10.0, NaCl 10.0, pH 7.0 (20.0 agar added to solid medium). Sterilization condition of medium: 1×10 5 Pa, sterilize for 30min.
[0051] Recombinant bacteria construction: the carbonyl reductase pure enzyme gene cr2 (amino acid sequence genebank: AB183149) from Candidamacedoniensis AKU4588 was obtained from the full-length DNA fragment by PCR based on the gene sequence, and the carbonyl reductase gene cr2 was respectively treated by NdeI and XhoI double enzyme digestion DNA fragment and expression vector pET21c, using ligase to connect the cohesive ends of the DNA fragment of the target gene and the vector fragment, obtain the recombinant plasmid pET21c-cr2, and further transform the competent Escherichia coli E.coliBL21 to obtain the recombinant strain E.coliBL21 / pET21c-cr2 .
[0052] Pick a single colony of E.coliBL21 / pET21c-cr2 and inoculate ...
Embodiment 2
[0054] Embodiment 2: the optimal pH value of recombinant carbonyl reductase CR2
[0055] Enzyme activity assay system: 100μL system, 0.5mmol / L NAD(P)H, 5mmol / L substrate, 30°C constant temperature for 3min, finally add 20U / L pure enzyme solution and mix well, start scanning the change of absorbance value at 340nm.
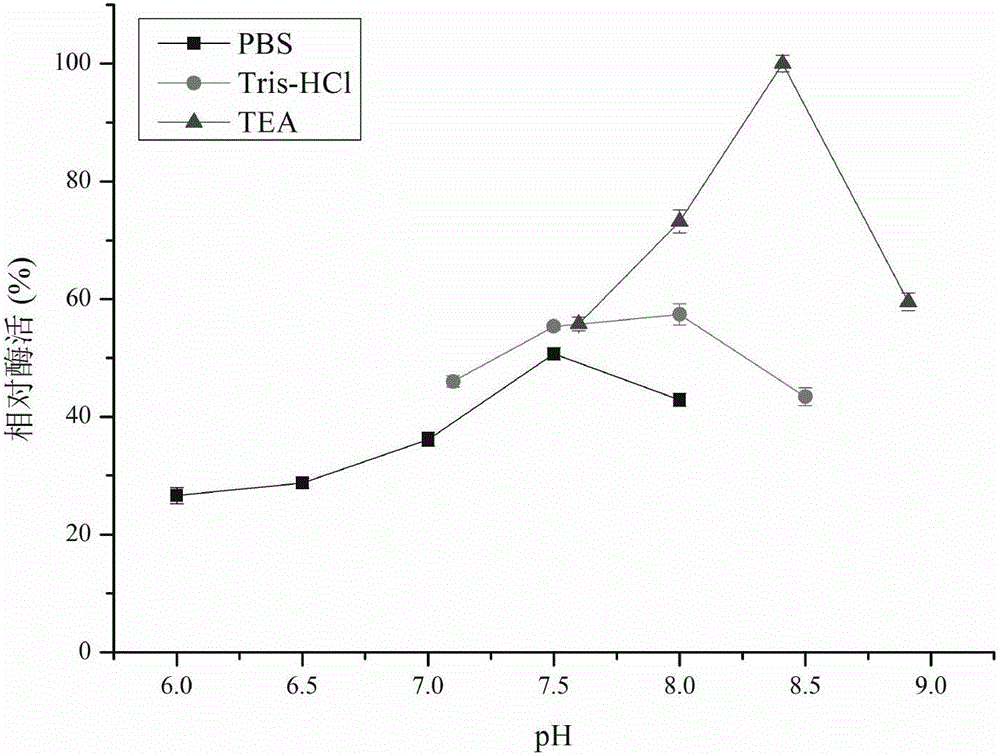
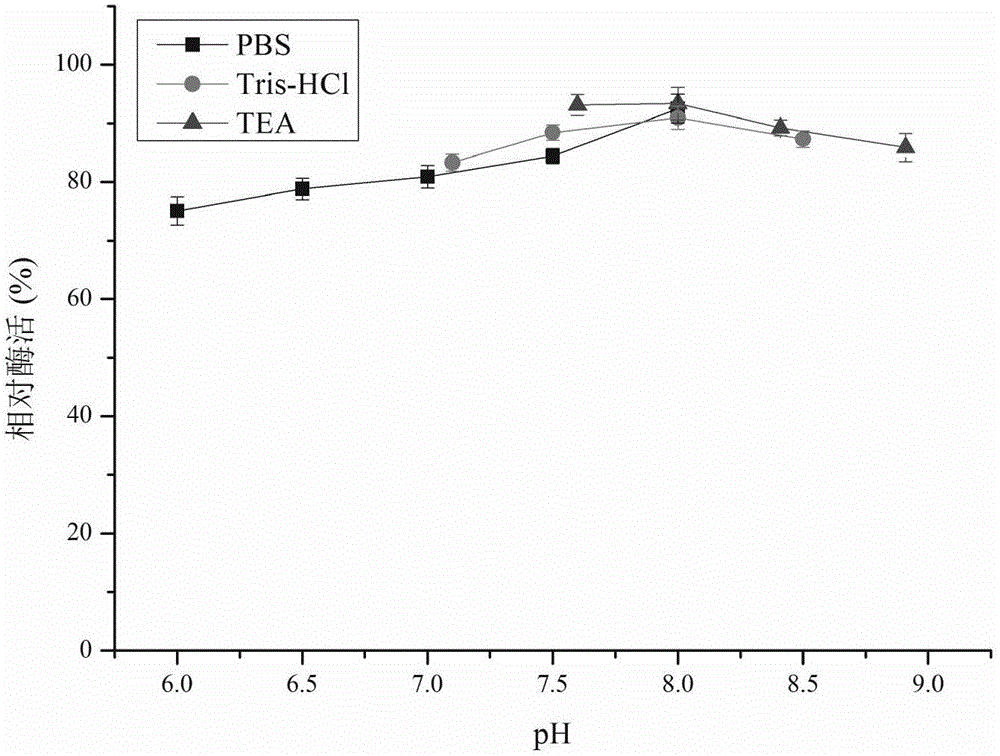
[0056] The relative enzymatic activity of recombinant carbonyl reductase CR2 was determined under different pH gradients (6.0-9.0) ( figure 1 ), the buffer used is 0.1mol / L phosphate buffered saline (PBS) (pH6.0-8.0), 0.1mol / L Tris-HCl buffer (pH7.0-8.5) and 0.1mol / L triethanolamine buffer ( TEA) (pH7.5-9.0). The research results show that the pH value has a greater impact on the enzyme activity of CR2 in the range of less than 7.0; between pH7.5-9.0, the relative enzyme activity of CR2 is above 50%; at pH8.4 (0.1mol / LTEA) CR2 has the highest catalytic activity of DKTP.
Embodiment 3
[0057] Embodiment 3: the optimum temperature of recombinant carbonyl reductase CR2:
[0058] Enzyme activity assay system: 100μL system, 0.1mol / L Tris-HCl buffer (pH7.5), 0.5mmol / L NAD(P)H, 5mmol / L substrate, kept at different temperatures for 3min, and finally added 20U / L pure enzyme After the liquid is mixed evenly, start to scan the change of absorbance value at 340nm.
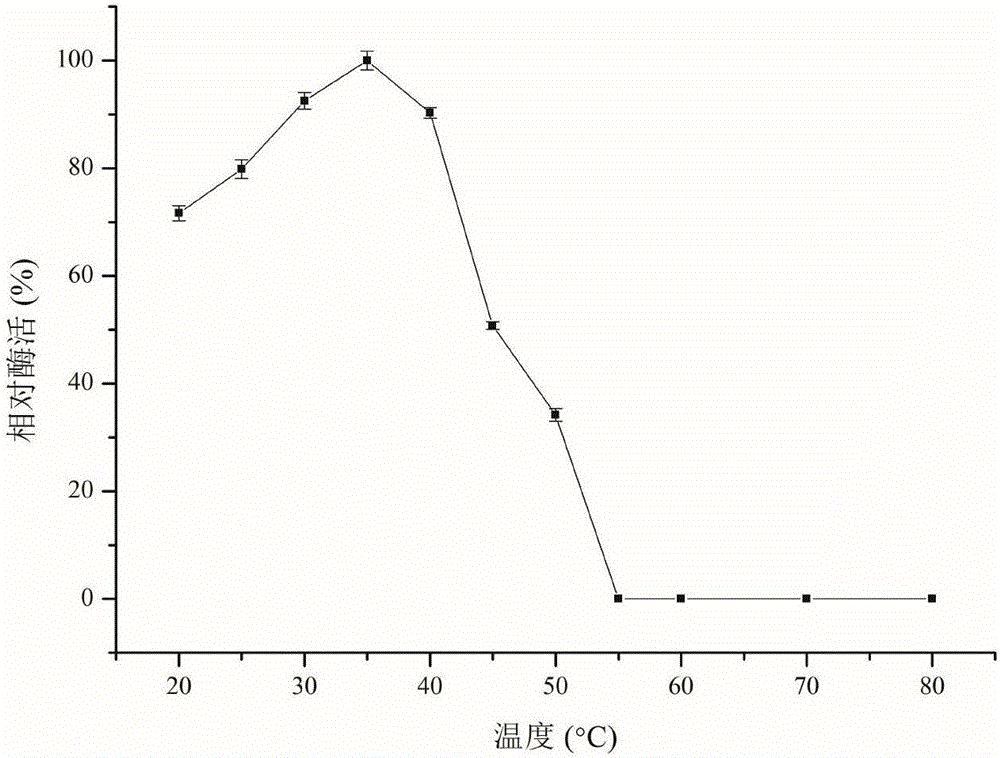
[0059] The relative enzymatic activity of recombinant carbonyl reductase CR2 was determined under different temperature gradients (20-80°C) ( figure 2 ). The study found that in the range of 25-40°C, the relative activity of CR2 was higher and remained above 80%; in the range of 20-35°C, as the temperature increased, the activity of CR2 continued to increase, and at 35°C The activity reached the maximum; when the temperature was higher than 40°C, the activity of CR2 began to decrease, and when the temperature was higher than 55°C, it was completely inactivated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com