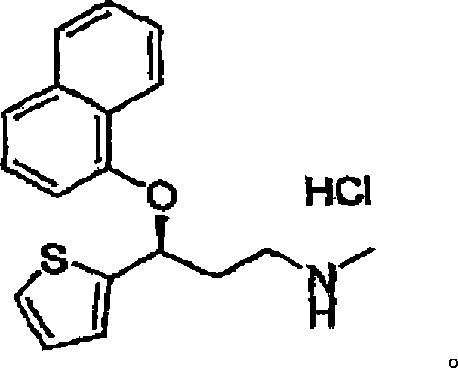
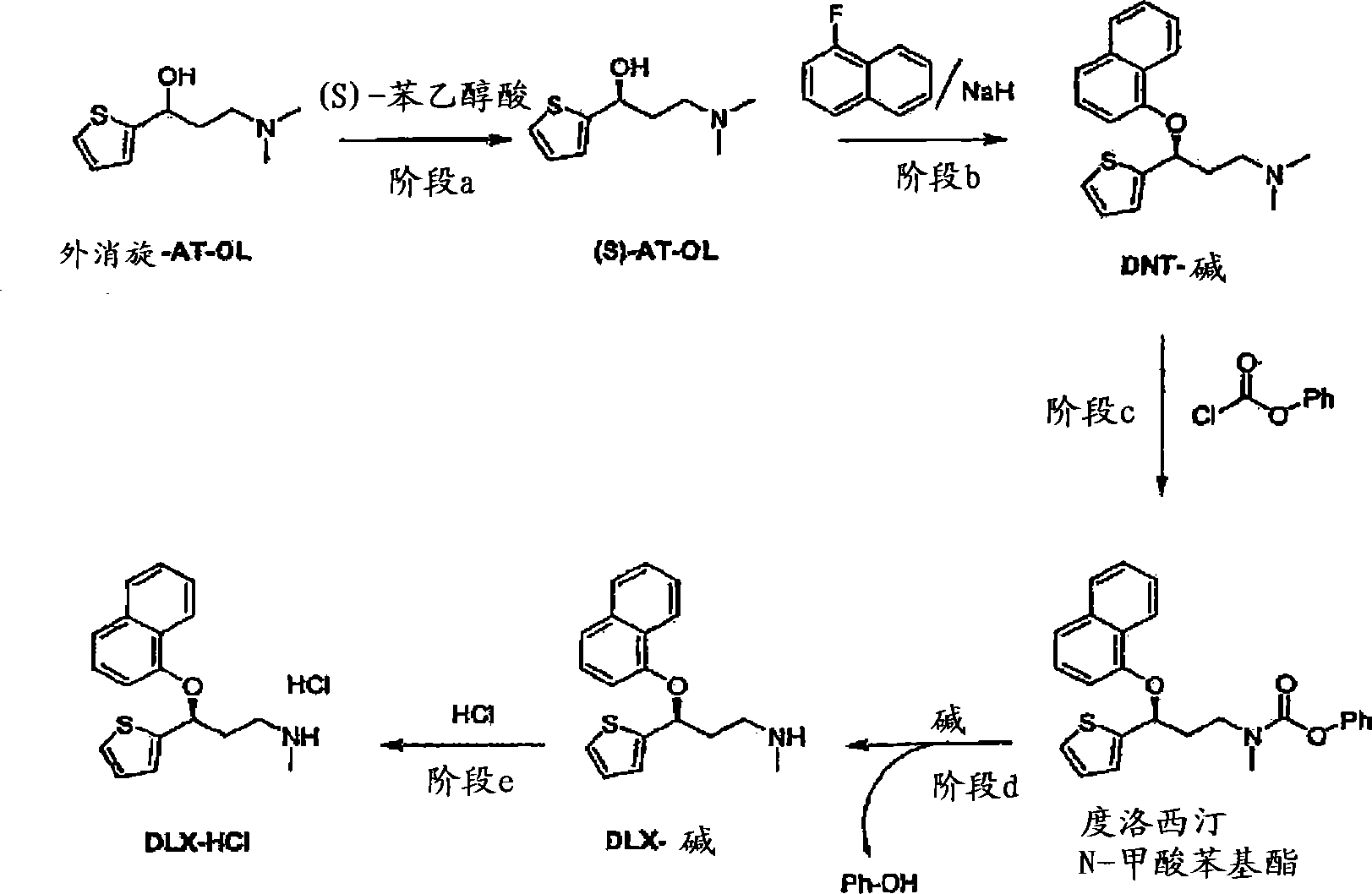
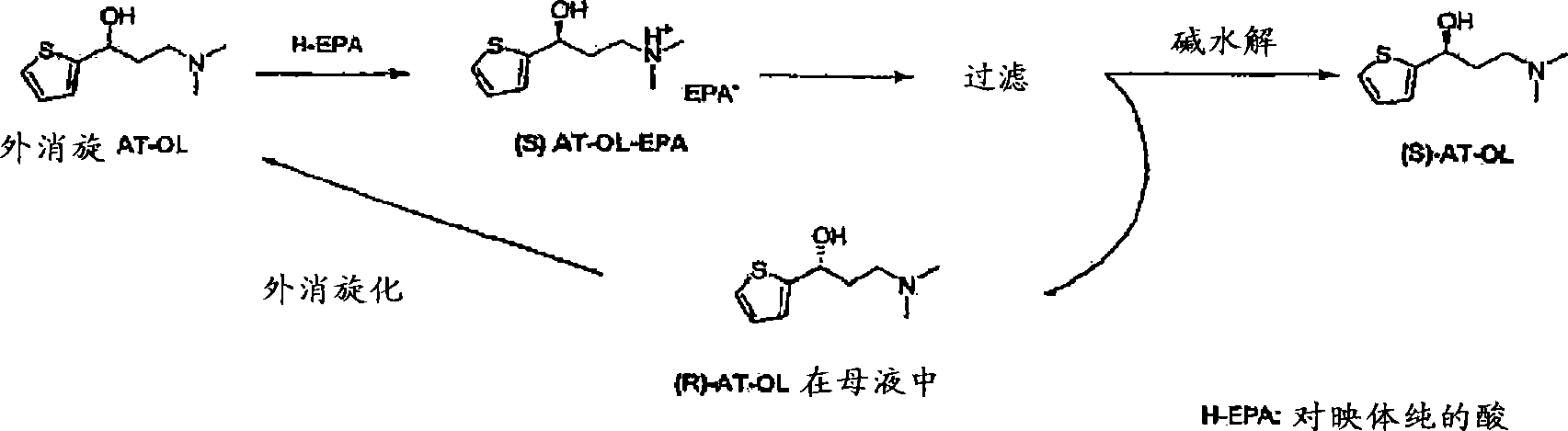
Process for the preparation of (s)-(-)-n,n-dimethyl-3-(2-thienyl)-3-hydroxypropanamine, a duloxetine intermediate
A technology of -AT-OL and phenylglycolic acid salt, applied in the field of preparing duloxetine intermediates, can solve problems such as shortage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1: Repeat chiral resolution of Preparation 1 in US 5362886 (2x scale)
[0072] The chiral resolution of AT-OL with mandelic acid in MTBE / ethanol was repeated (Repeat 1 in US 5,362,886). The enantiomer R level was measured to be 7.01%.
[0073] A solution of 8.2 g of (S)-mandelic acid in 25 ml of ethanol (heated to dissolve at 50°C) was added to a solution of 20 g of (R,S)-AT-OL in 300 ml of MTBE at 50°C. The resulting mixture was heated to reflux for 45 minutes, then cooled to room temperature and stirred overnight (1 hour in this patent). The resulting solid was filtered and dried in a vacuum oven to give 16 g of (S)-AT-OL mandelic acid salt (enantiomer R: 7.01%).
Embodiment 2
[0074] Example 2: Chiral resolution of AT-OL in IPA
[0075] A solution of 2 g (S)-mandelic acid in 10 ml IPA (heated to dissolve at 50°C) was added to a solution of 5 g (R,S)-AT-OL in 40 ml IPA at 50°C. The resulting mixture was heated to reflux for 45 minutes and then cooled to room temperature. The resulting solid was filtered and dried in a vacuum oven to yield 3.5 g of (S)-AT-OL mandelic acid salt (enantiomer R: 15.03%).
Embodiment 3
[0076] Example 3: Chiral resolution of AT-OL in MIBK
[0077] A solution of 2 g (S)-mandelic acid in 10 ml MIBK (heated to dissolve at 50° C.) was added to a solution of 5 g (R,S)-AT-OL in 10 ml MIBK at 50° C. The resulting mixture was heated to reflux for 45 minutes and then cooled to room temperature. The resulting solid was filtered and dried in a vacuum oven to yield 3.8 g of (S)-AT-OL mandelic acid salt (enantiomer R: 3.87%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com