Duloxetine formulation
A technology for duloxetine and preparations, which is applied in the field of duloxetine preparations, and can solve problems such as insolubility, reduced bioavailability of active substances, and slow dissolution
Inactive Publication Date: 2010-03-31
箭锋国际有限公司
View PDF2 Cites 7 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0004] However, duloxetine is also known to react with many known enteric coatings, forming a slowly dissolving or even insoluble coating, reducing the bioavailability of the active substance
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 9
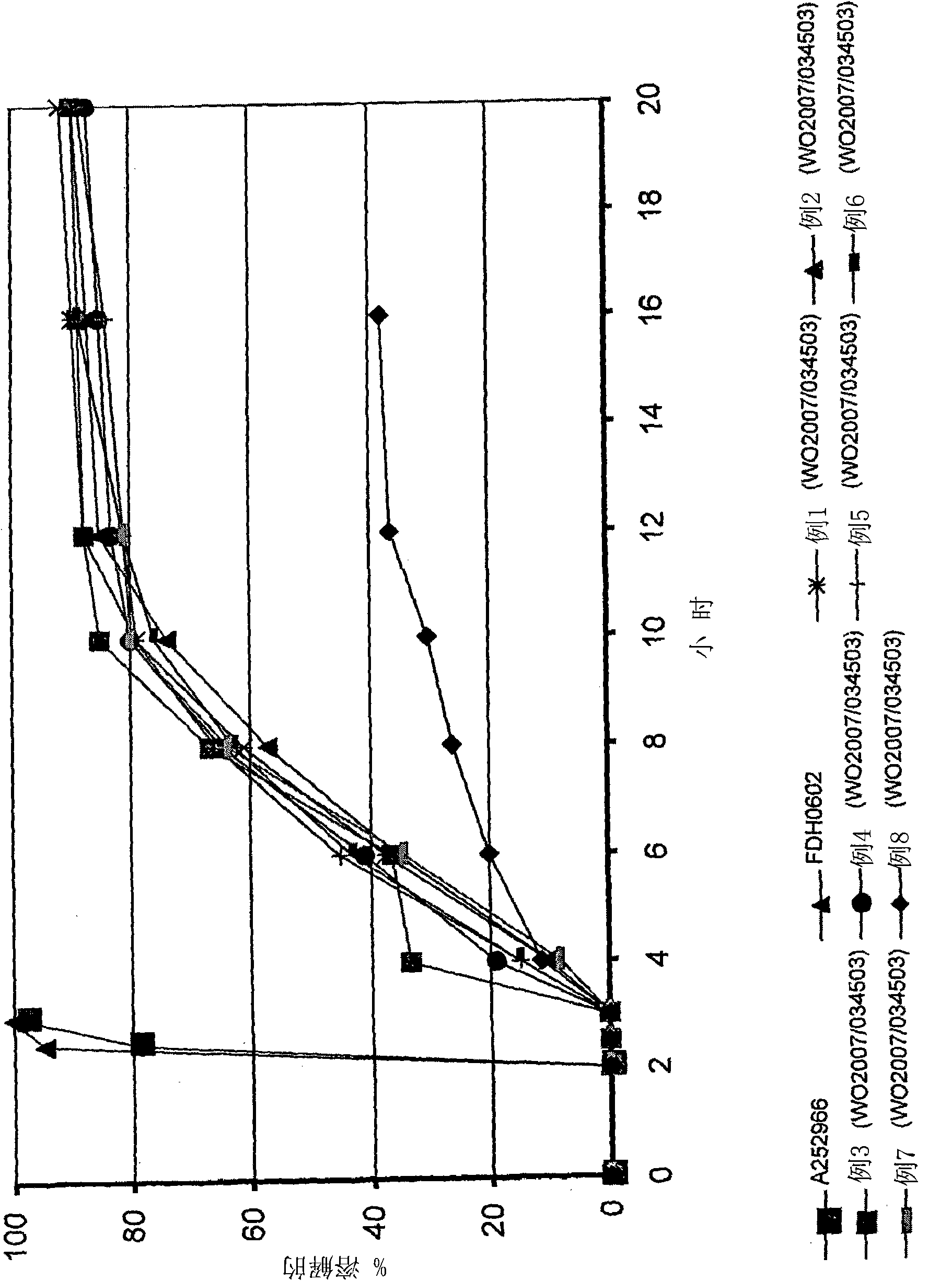
[0079] The following studies were carried out to demonstrate the efficacy of the formulation of the invention (FDH0602) compared to the known commercially available brand (Cymbalta, A2523966) and the controlled release formulation of duloxetine disclosed in WO2007 / 034503 (Examples 1-8 below). release curve.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
Login to View More
Abstract
A duloxetine pellet formulation comprises: (i) a core including a desired amount of duloxetine; (ii) an enteric coating comprising hydroxypropylmethylcellulose phthalate (HPMCP) as an enteric polymer;and, optionally, (iii) a separating layer located between the core and the enteric coating, the separating layer including polyvinyl alcohol and a low molecular weight hydroxypropylmethylcellulose (HPMC).
Description
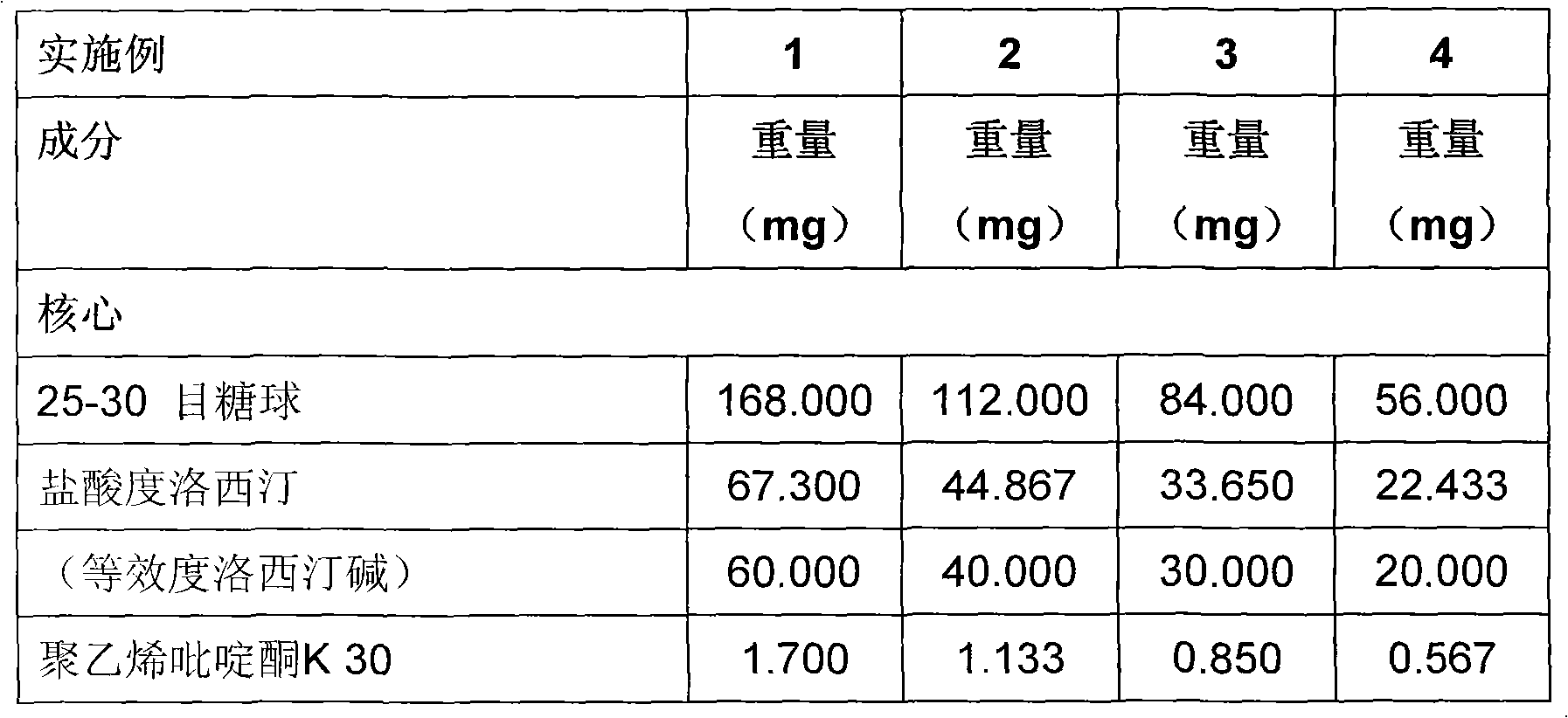
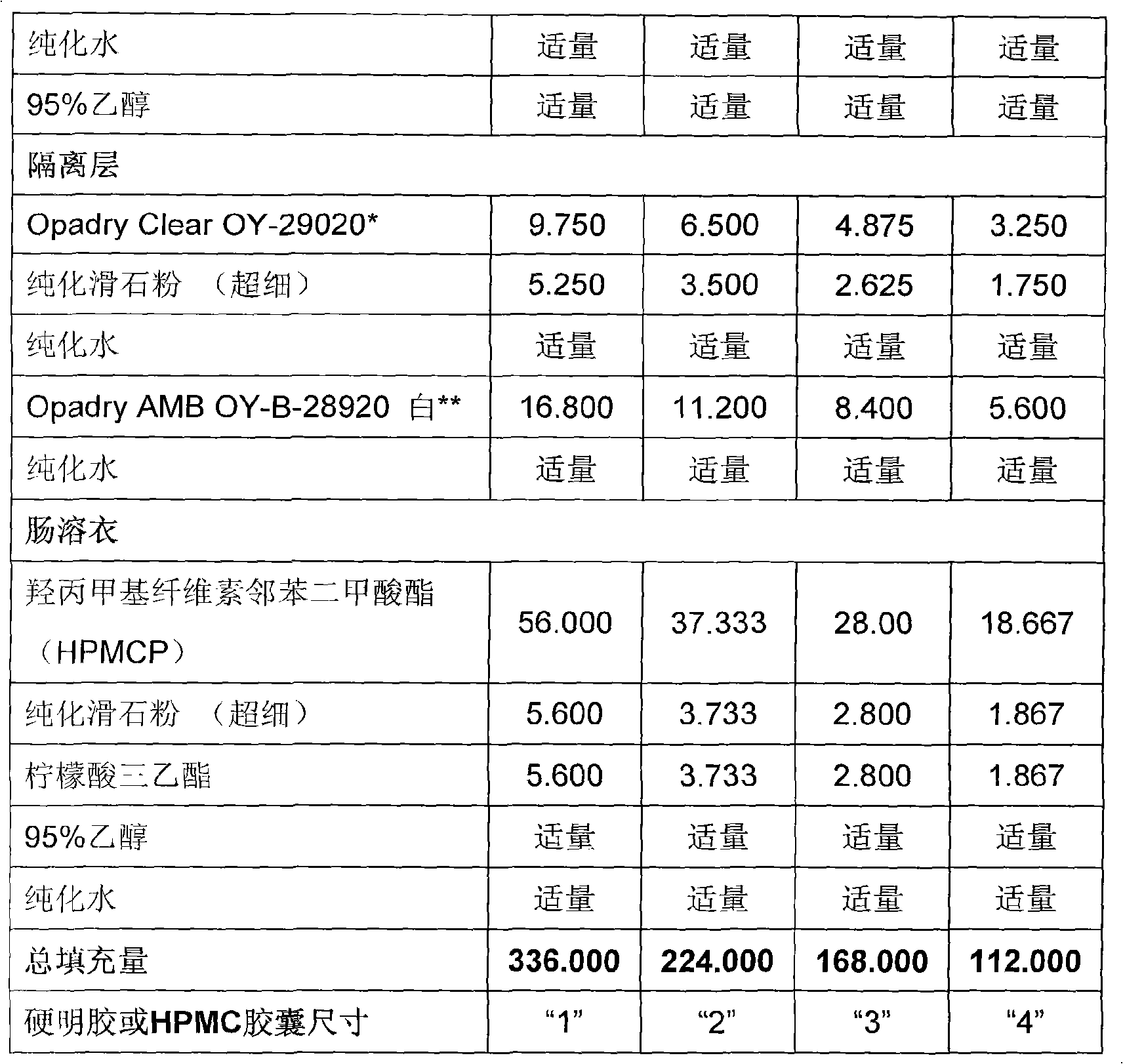
technical field [0001] The invention relates to an active substance duloxetine pharmaceutical preparation and a preparation process thereof. Background technique [0002] Duloxetine: (+)-(S)-N-methyl-y-(1-naphthyloxy)-2-thienylamine hydrochloride is an SSNRI (selective serotonin and norepinephrine reuptake inhibitor ) is a well-known drug in the class of compounds, which can be used in the treatment of depression MDD, diabetic peripheral neuralgia and incontinence, especially stress urinary incontinence (SUI). The hydrochloride salt is commonly used, and the term "duloxetine" is used hereinafter to refer generally to duloxetine free base, but is also used to refer to duloxetine salts and other duloxetine compounds, But generally refers to its hydrochloride. [0003] Duloxetine is known to be unstable in acidic media. Therefore, there is a need to protect the active substance thereof from the acidic environment of the stomach and to allow its release in the gastrointestina...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K9/36A61K9/62A61K31/381A61P25/24
CPCA61K9/5078A61K9/5042A61K9/2886A61K31/381A61K9/5047A61P13/10A61P25/24A61P43/00
Inventor R·萨林P·H·R·珀西卡纳
Owner 箭锋国际有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com