Alcohol dehydrogenase and uses thereof in synthesis of Duloxetine intermediate
An alcohol dehydrogenase and reaction technology, applied in the field of bioengineering, can solve the problems of incomplete reaction, low yield, high cost of raw materials and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
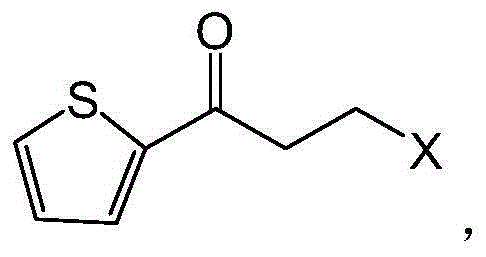
[0048] Collect soil samples from Zhujia Village, Situan Town, Fengxian District, Shanghai and extract DNA (extraction method refers to ChromaSpinTE-1000, Clontech Laboratories, Inc., USA), partially digest with Sau3AI, collect 2-8kb fragments by electrophoresis, recover and connect to BamHI site of pUC19 to obtain a plasmid library. Transform the library into E.coliDH5α and smear it on an LB plate containing 100 μg / mL ampicillin. Select positive clones and transfer them to a 96-deep-well plate with 500 μg / mL LB (100 μg / mL ampicillin). After culturing at 37°C for 4 hours, add 1 mMIPTG Induction, continue to culture overnight at 30°C; then take 50 μL of deep-well culture each to a new 96-well plate added with 50mM sodium phosphate buffer (pH7.5), freeze and thaw repeatedly at -80°C to lyse the bacteria; add 1mM duloxetine Substrate DKTP, 10mM glucose, 1 unit of glucose dehydrogenase, 0.002% (v / v) phenol red, cultured at 30°C for 4 hours, picked the deep well culture correspondin...
Embodiment 2
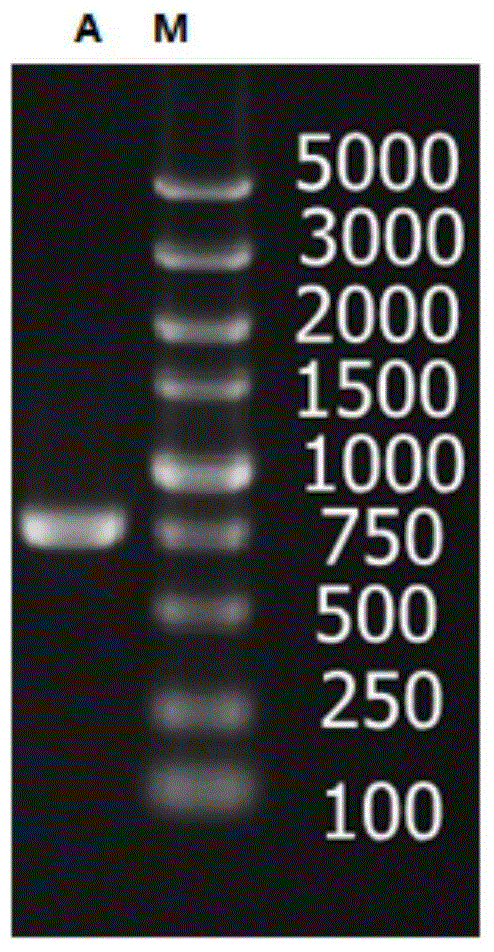
[0050] A primer pair P1 (nucleotide sequence: SEQ ID NO: 3) and P2 (nucleotide sequence: SEQ ID NO: 4) was synthesized. Use P1 and P2 to clone the full-length alcohol dehydrogenase gene, the PCR system is as follows: 10×KOD-Plus PCR buffer 2μL, 25mM MgSO 4 1.2 μL, 2 mMdNTP 2 μL, KOD-PlusPCR high-fidelity enzyme 0.3 μL, DNA template 0.5 μL (including 0.1 μg DNA template), ddH 2 O13μL, P1 and P2 each 0.5μL (10mmol / L). PCR amplification steps are: (1) 95°C, pre-denaturation for 3min; (2) 98°C, denaturation for 15s; (3) 58°C annealing for 30s; (4) 72°C extension for 1min; steps (2) to (4) repeated 30 times; (5) Continue extending at 72°C for 10 minutes, then cool to 4°C. The PCR product was purified by agarose gel electrophoresis, and the target band in the 700-800bp range was recovered using an agarose gel DNA recovery kit (see figure 1 ), obtained a complete gene sequence, which was 750bp in length after DNA sequencing.
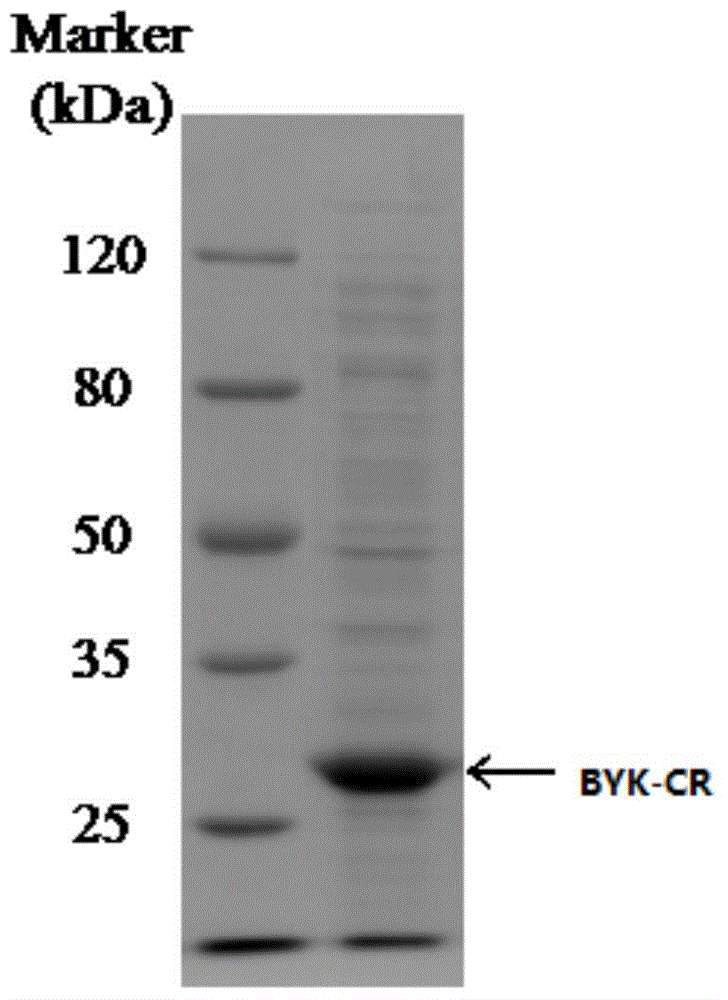
[0051] After the PCR product was cut and recovered, it ...
Embodiment 3
[0053] Enzyme activity determination: In 2mL reaction solution, add 2mM MDKTP as substrate, 0.1mM NADH as cofactor, then add 20μL crude enzyme solution, measure OD within 2 minutes 340 The rate of decrease is ΔA 340 . Enzyme activity calculation formula: ΔA 340 ×1000 / (6220×20), that is, the specific enzyme activity per mL of lysate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com