Method for preparing duloxetine
A technology of duloxetine and thienyl group, applied in the field of preparation of duloxetine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The following are specific examples of the present invention to further describe the technical solution of the present invention, but the present invention is not limited to the examples.
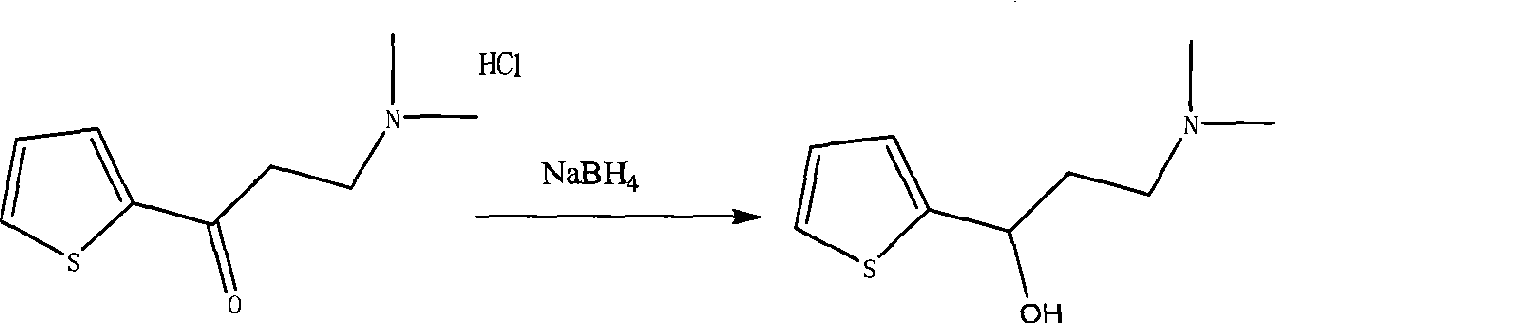
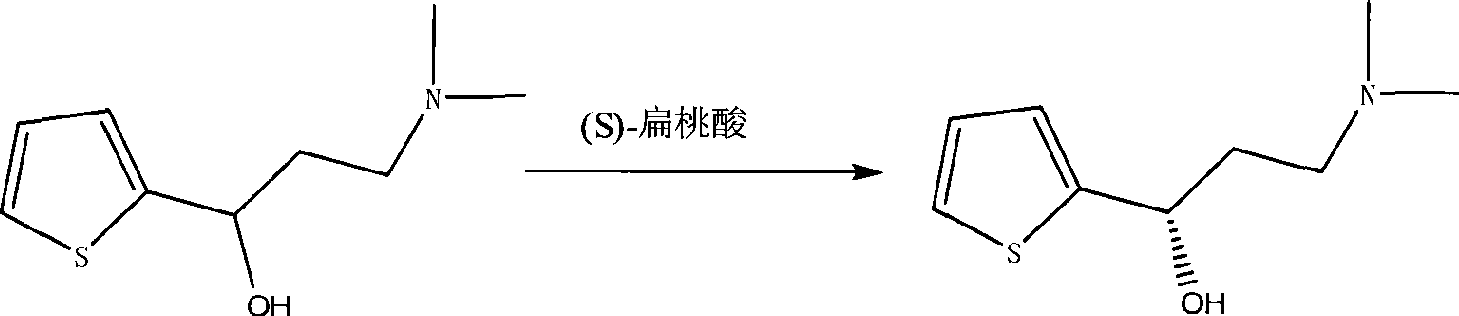
[0022] Duloxetine according to the present embodiment is prepared according to the following steps:
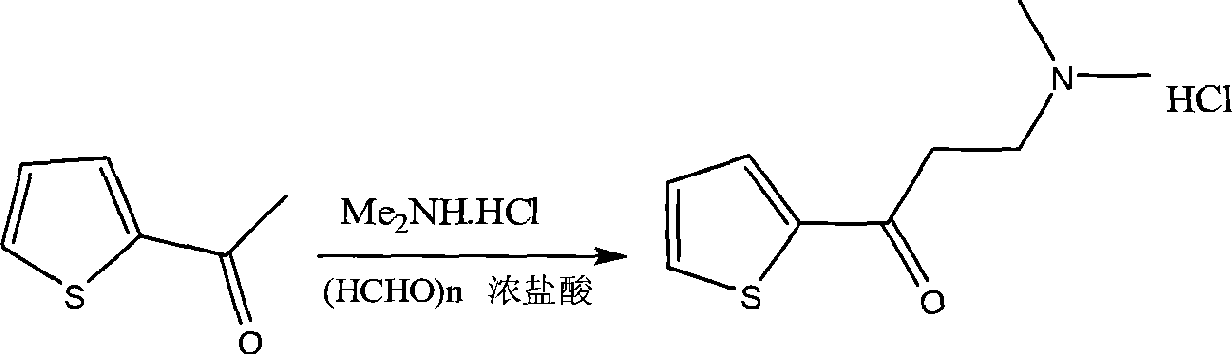
[0023] (1), Mannich reaction prepares 3-dimethylamino-1-(2-thienyl)-1-acetone hydrochloride, and the equation is as follows:
[0024]
[0025] Set up a device with mechanical stirring, put 100g of 2-acetylthiophene, 37.7g of paraformaldehyde, 91g of dimethylamine hydrochloride and 523ml of ethanol into the three-necked reaction flask in turn, start stirring, and gradually add 3.8ml of concentrated hydrochloric acid dropwise, After dripping, start the water bath to control the temperature to 83°C to reflux. After all the solids were dissolved, reflux was started, and a white solid was formed after 1 hour (at this time, attention should be paid to slowing down the stirring speed), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com