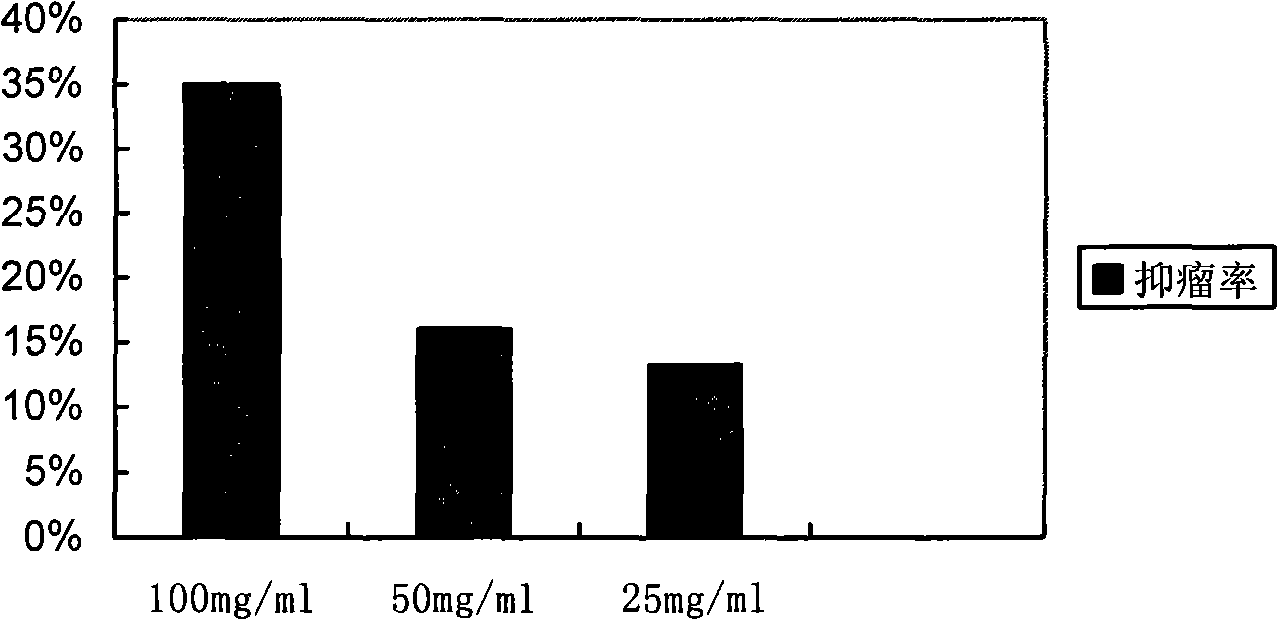
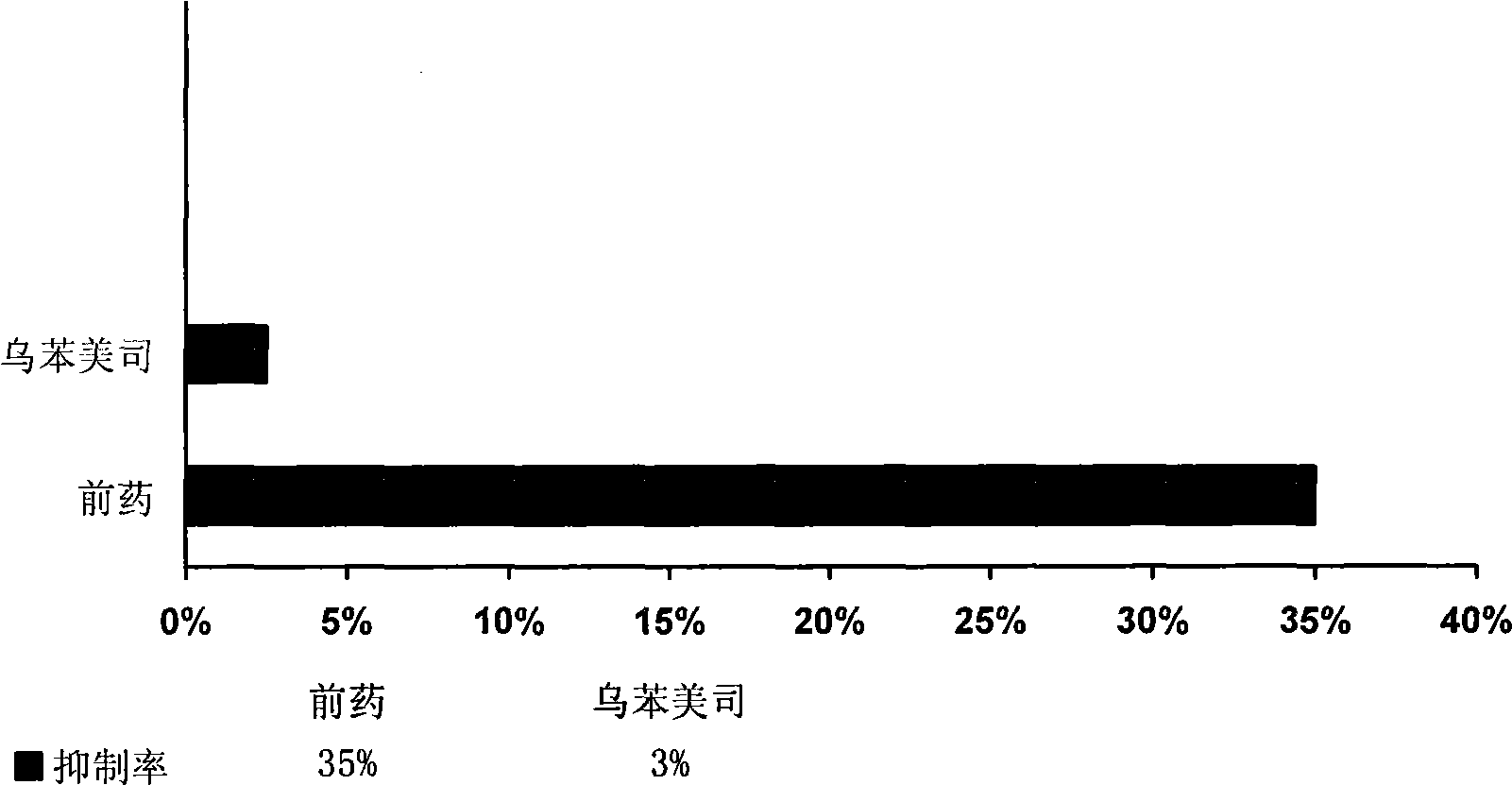
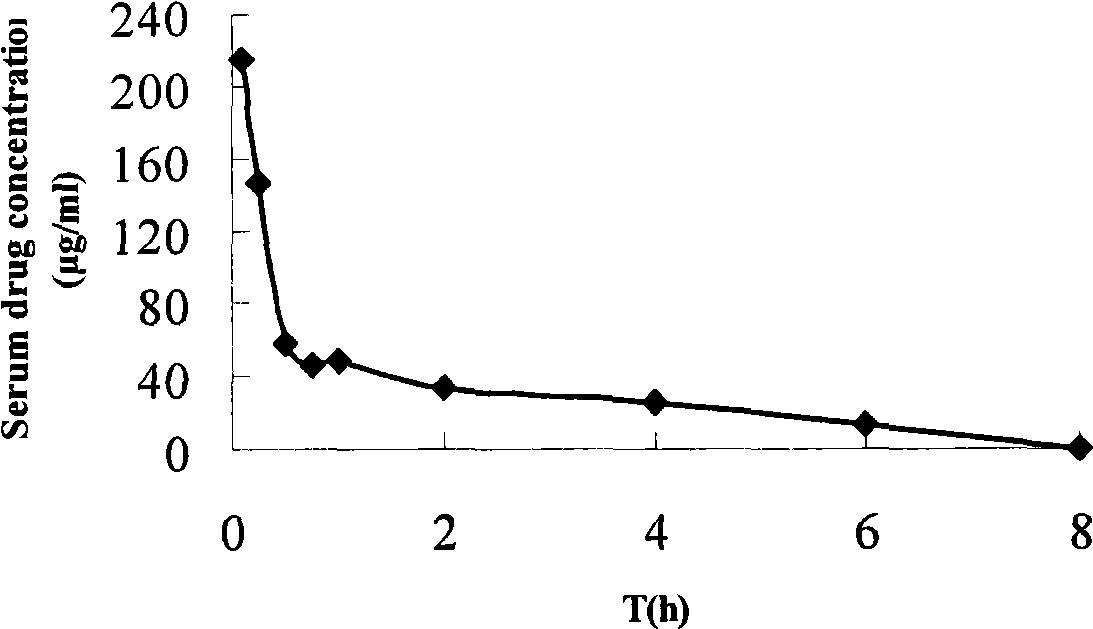
Aminopeptidase N inhibitor bestatin dino ester, synthesis and application thereof
A technology of ubenimestinol ester and ubenimex, which is applied in the direction of drug combination, carboxylic acid ester preparation, carboxylic acid amide preparation, etc., can solve the problems of poor solubility in various organic solvents, poor metabolic stability, and bioavailability. To improve the dynamic properties of the belt, increase water solubility, and significantly inhibit enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] An antineoplastic agent, ubenmesedinol, having the following structure:
[0125]
[0126] Synthetic route of uben mestinol ester:
[0127]
[0128] Uben mestinol synthetic method, the steps are as follows:
[0129] (1) With optically pure ubenimex as the starting material, under the alkaline condition of pH=9-10, ubenimex and di-tert-butyl dicarbonate are reacted (the molar amount of di-tert-butyl dicarbonate 1.1 times that of Ubenimex), the primary amino group of Ubenimex is Boc-protected,
[0130] (2) Using anhydrous tetrahydrofuran as a solvent, under the catalysis of 0°C temperature and dicyclohexylcarbodiimide (DCC) (the molar weight of DCC is 1.1 times that of Ubenimex), the product of step (1) Boc-protected ubenimex condenses with HOBt (1.08 times the molar weight of ubenimex) to form an active ester,
[0131] (3) The product active ester reflected in the previous step reacts with N'N dimethylethanolamine at room temperature under the catalysis of p-dime...
Embodiment 2
[0134] Accurately weigh sodium chloride according to the prescription amount, add 70% of the prescription amount to dissolve in water for injection, add 1% active carbon by volume, stir, filter, HCl to adjust pH5, add the ubene medinolate of the prescription amount embodiment 1 , stir to dissolve, add water for injection to 1000ml, filter, fill in 2ml ampoules, and sterilize for 30 minutes.
Embodiment 3
[0136] Accurately weigh glucose according to the prescription amount, add 70% of the prescription amount in water for injection to dissolve, add 1% activated carbon by volume, stir, filter, adjust pH5 with HCl, add the ubene mesidinol ester of the prescription amount embodiment 1, and stir Dissolve, add water for injection to 1000ml, filter, fill in 2ml ampoules, and sterilize for 30 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com