Raav vectors for the treatment of gm1 and gm2 gangliosidosis
A lysosomal storage disease, capsid technology, applied in the field of RAAV vectors for the treatment of GM1 and GM2 gangliosidosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0231] Example 1: Materials and Methods
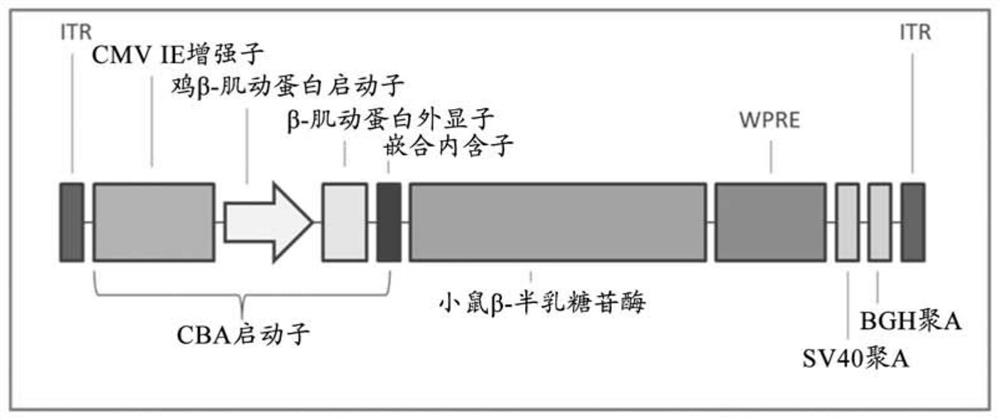
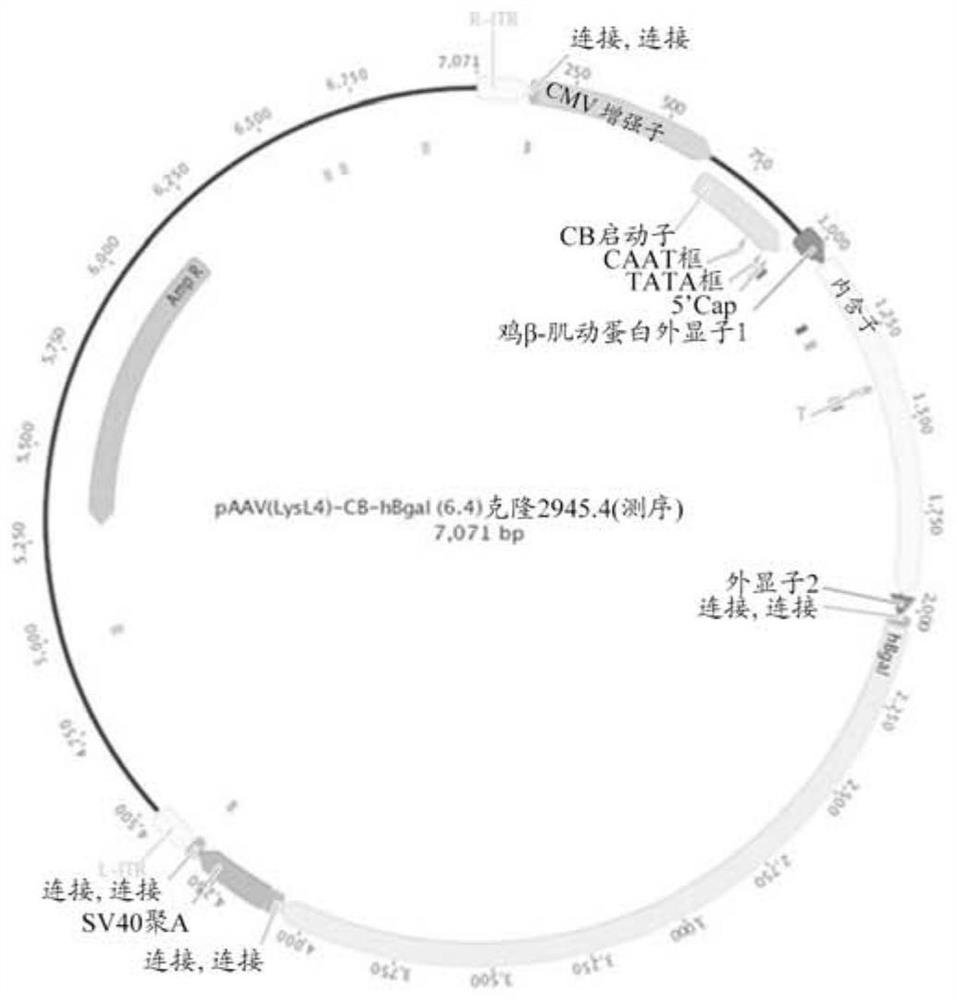
[0232] Vector design, construction and virus creation.
[0233] An AAV vector (SEQ ID NO: 3) was constructed and carried an expression cassette driven by a promoter, followed by a chimeric chicken beta-actin / rabbit beta globin intron (CBA), a mouse lysate Enzyme acid β-galactosidase cDNA (mβgal), woodchuck hepatitis virus post-transcriptional regulatory element (WPRE), and two polyA signals in tandem derived from bovine growth hormone (BGH) and SV40, the promoter by Consists of the cytomegalovirus immediate early enhancer (CMV) fused to the chicken β-actin promoter. This vector is called AAV-CBA-mβgal-WPRE. AAV-CBA-mβgalE269Q-WPRE was generated by PCR mutagenesis using the following primers: For 1 : AAA CGT CTC ACT AGT CCG CGG AAT TC (SEQ ID NO: 7), Rev 1 : AAACGT CTC ACT GAG AAT TGA TCA AA (SEQ ID NO: 7) : 8), For2: AAA GGT CTC CGG CCG CTA GCGTCA G (SEQ ID NO: 9), Rev2: AAA GGT CTC ATC AGT TCT ATA CTG GC (SEQ ID NO: 10). The res...
Embodiment 2
[0261] AAV dose-dependent distribution of βgal in the brain
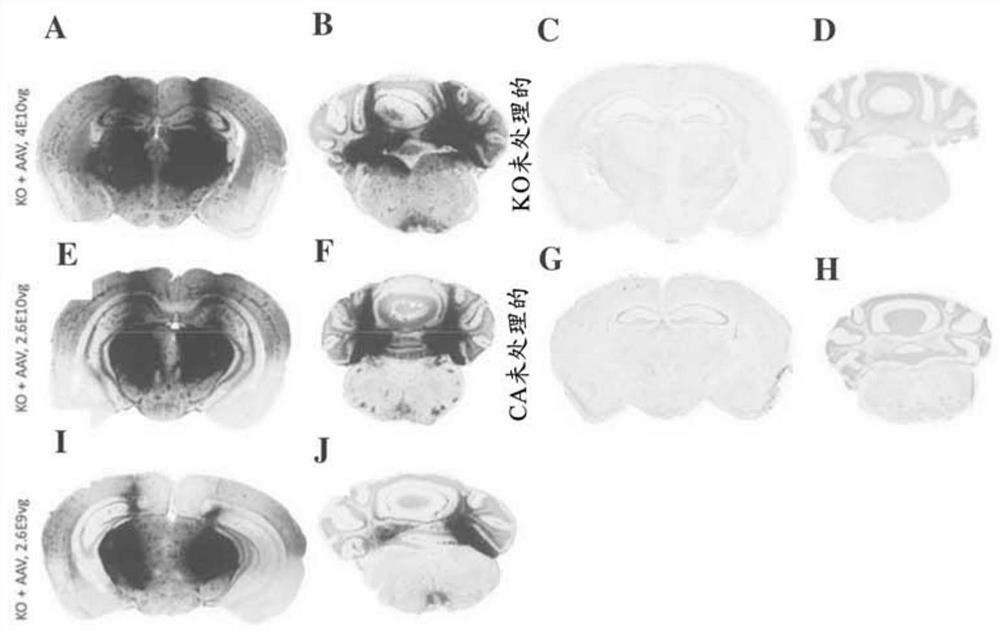
[0262] The AAVrh8-CBA-mβgal-WPRE vector (Figures 1 and 20) was infused into 6-8 week old GM1 gangliosides by bilateral injection in the thalamus and deep cerebellar nucleus at total doses of 4e10vg, 2.6e10vg and 2.6e9vg, respectively storage disease mice (βgal - / - ) in the brain. Animals in the highest dose cohort received bilateral injections of 1 μl in the thalamus and DCN, while animals in the other two cohorts received 1 μl in the thalamus and 0.3 μl in the DCN. The βgal distribution pattern in the brain at 3 months post-injection appears to be dose-dependent, with the highest intensity of gal activity at the injection site ( figure 2 ). The highest dose (4e10vg) in the brain ( figure 2 A) and the cerebellum ( figure 2 Enzyme activity is provided within most of the sections in B). Moderate doses of 2.6e10vg had similar activity levels in the brain ( figure 2 E), while the cerebellum ( figure 2 F) ...
Embodiment 3
[0266] AAV treatment extends life
[0267] with untreated βgal - / - AAVrh8-treated βgal compared to control - / - Mice lived significantly longer ( Figure 4 ). untreated βgal - / - Median survival was 245.5 days for controls (N=18), 293.5 days for the 4e10vg arm (N=12, p=0.0004), 349 days for the 2.6e10vg arm (N=13, p=0.002), and 2.6e9vg arm was 389 days (N=12, p<0.0001).
[0268] GM1 ganglioside storage persists in the injection site and spinal cord of AAV-treated long-lived animals
[0269] Histological analysis of lysosomal storage by non-Ruppin staining in the animals' CNS 3 months post-injection revealed an almost complete correction in the brain and cerebellum corresponding to the presence of the enzyme, as Xgal staining at the same time point see in ( figure 2 ). Surprisingly, non-rapine-positive cells were found only at the injection site or along the injection track ( Figure 5 C, 5E).
[0270] βgal injection of AAVrh8 - / - The presence of nonruppin-positiv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com