Quinazolinone-containing diaryl urea compound and preparation method and application thereof
A technology of diaryl urea and quinazolinone, which is applied in the field of biomedicine, can solve the problems of affecting the treatment of patients, aggravating the pain and burden of patients, and achieve the effect of reducing anaphylactoid reactions, reducing pain and burden, and cheap reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
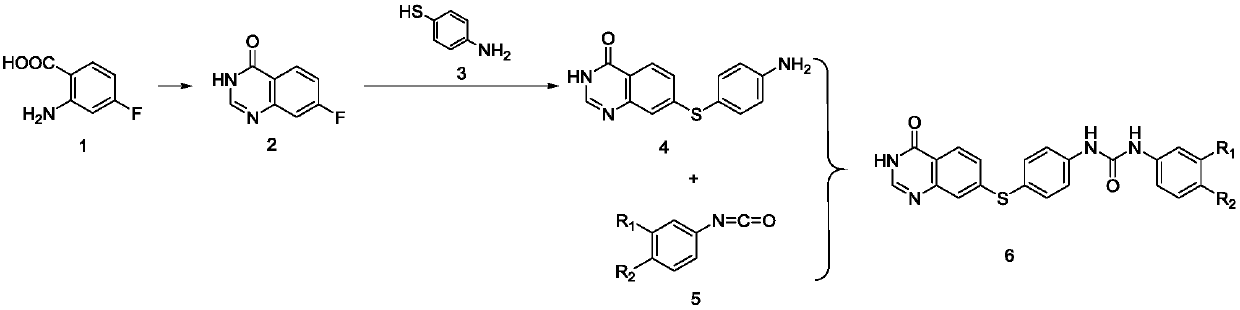
[0040] The preparation method 1 of the described diaryl urea compound containing quinazolinone comprises the following steps:
[0041] 1) 2-amino-4-fluorobenzoic acid and formamide prepare 7-fluoroquinazolin-4(3H)-one by ring closure reaction;
[0042] 2) Preparation of 7-((4-aminophenyl)thio)quinazolin-4(3H)-one by nucleophilic substitution reaction between 7-fluoroquinazolin-4(3H)-one and p-aminothiophenol;
[0043] 3) Nucleophilic reaction of phenyl isocyanate with substituent and 7-((4-aminophenyl)thio)quinazolin-4(3H)-one to obtain diaryl urea compound containing quinazolinone.
[0044] Preferably, in step 1) of Preparation Method 1, 2-amino-4-fluorobenzoic acid is dissolved in formamide and reacted at 160°C under nitrogen protection. After the reaction is completed, the reaction solution is poured into ice water and filtered. The filter cake was recrystallized from methanol to give 7-fluoroquinazolin-4(3H)-one.
[0045] Preferably, in step 2) of Preparation Method 1, 7...
Embodiment 1
[0057] 1) Preparation of 7-fluoroquinazolin-4(3H)-one (compound 2) by cyclization reaction of 2-amino-4-fluorobenzoic acid (compound 1) and formamide;
[0058] Weighed 10.0 g of compound 1, dissolved it in 30 mL of formamide, and reacted at 160°C for 6 h under nitrogen protection. After the reaction, the reaction solution was poured into ice water, filtered, and the filter cake was recrystallized with methanol to obtain compound 2.
[0059] 2) Preparation of 7-((4-aminophenyl)thio)quinazoline-4 by substitution reaction between 7-bromoquinazolin-4(3H)-one (compound 2) and p-aminothiophenol (compound 3) (3H)-ketone (compound 4);
[0060] Compound 2 2.0 g, K 2 CO 3 1.26g was added to 10mL dimethyl sulfoxide, under argon protection, the temperature was raised to 115°C; a solution of 1.14g of p-aminothiophenol 3 in dimethyl sulfoxide (5mL) was added dropwise to the above reaction solution, and reacted for 1h After the reaction, the reaction solution was poured into ice water, e...
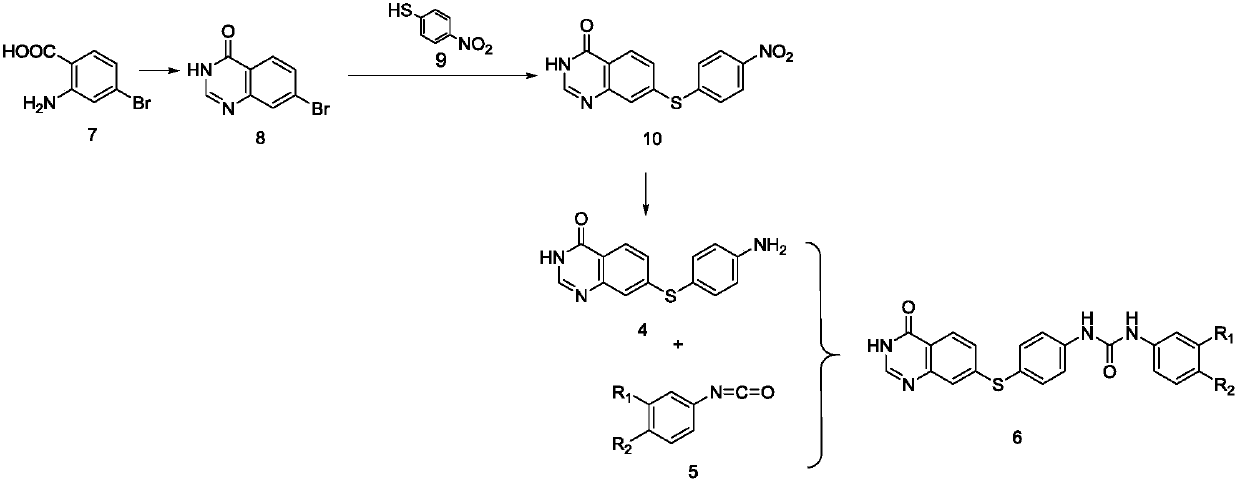
Embodiment 2
[0064] 1) Preparation of 7-bromoquinazolin-4(3H)-one (compound 8) by cyclization reaction of 2-amino-4-bromobenzoic acid (compound 7) and formamide;
[0065] Weigh 5.1g of compound 7 in a 250mL round bottom flask with an analytical balance, and add 100mL of formamide solution, N 2 Microwave reaction under protection (150°C, 1.45h); after the reaction, ice water was added while it was hot, extracted with ethyl acetate, the organic phase was concentrated and stood to precipitate solid, and suction filtered. The obtained filter cake is compound 8.
[0066] 2) Preparation of 7-((4-nitrophenyl)thio)quinazoline-4 by reacting 7-bromoquinazolin-4(3H)-one (compound 8) with p-nitrothiophenol (compound 9) (3H)-ketone (compound 10);
[0067] Compound 8 0.5g, p-nitrothiophenol 1.02g, K 2 CO 3 Add 0.3g and 0.02g of CuI into a 100mL round-bottomed flask. Under the condition of ice bath, vacuum nitrogen protection, then raise the temperature to 130°C, and react for 6h;
[0068] 3) 7-((4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com