Diaryl urea compounds containing quinazolinone and preparation method and application thereof
A technology of diaryl urea and quinazolinone, which is applied in the field of biomedicine, can solve the problems of affecting the treatment of patients, aggravating the pain and burden of patients, and achieve the effect of reducing anaphylaxis, alleviating pain and burden, and cheap reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
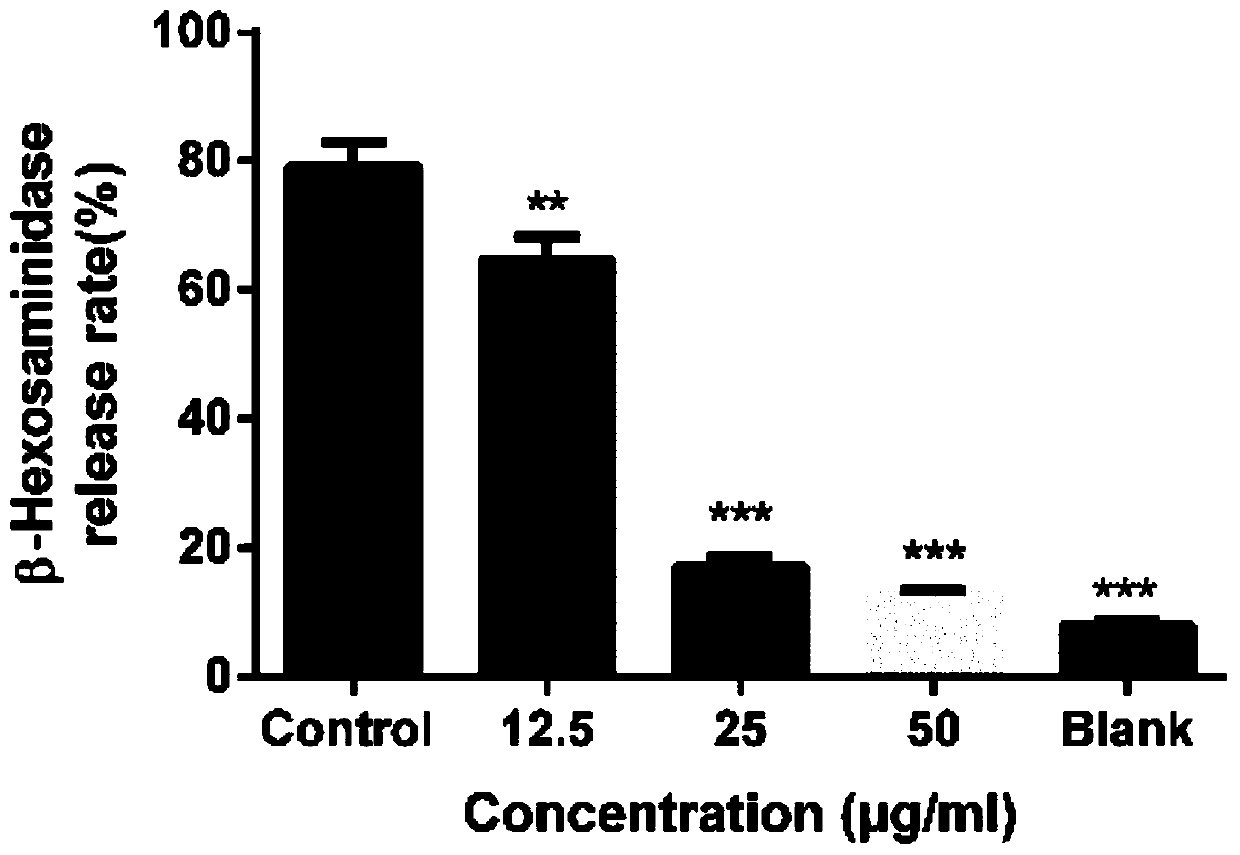
Image
Examples
preparation example Construction
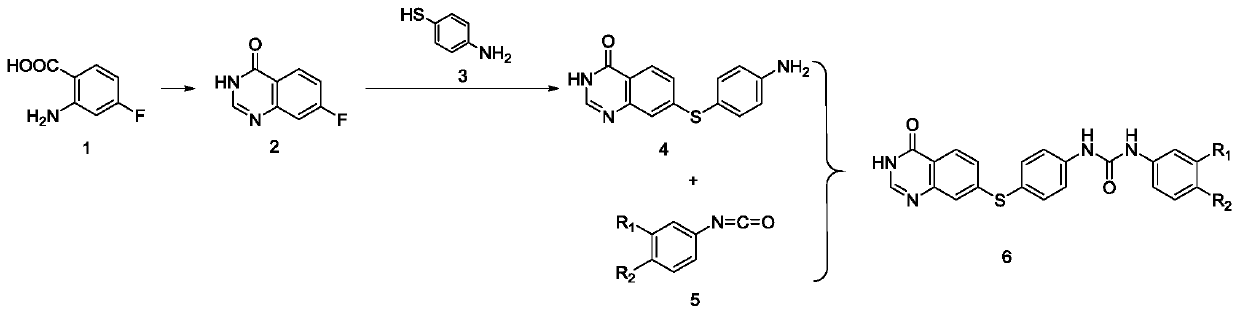
[0040] The preparation method 1 of the diarylurea compound containing quinazolinone includes the following steps:
[0041] 1) 2-Amino-4-fluorobenzoic acid and formamide are cyclized to prepare 7-fluoroquinazolin-4(3H)-one;
[0042] 2) Preparation of 7-((4-aminophenyl)thio)quinazolin-4(3H)-one by nucleophilic substitution reaction between 7-fluoroquinazolin-4(3H)-one and p-aminothiophenol;
[0043] 3) Diarylurea compounds containing quinazolinones are obtained by nucleophilic reaction between phenyl isocyanate containing substituents and 7-((4-aminophenyl)thio)quinazolin-4(3H)-one.
[0044] Preferably, in step 1) of preparation method 1, 2-amino-4-fluorobenzoic acid is dissolved in formamide and reacted at 160°C under nitrogen protection. After the reaction is completed, the reaction solution is poured into ice water and filtered, The filter cake was recrystallized from methanol to obtain 7-fluoroquinazolin-4(3H)-one.
[0045] Preferably, in step 2) of Preparation Method 1, 7-fluoroquin...
Embodiment 1
[0057] 1) 2-Amino-4-fluorobenzoic acid (compound 1) and formamide are cyclized to prepare 7-fluoroquinazolin-4(3H)-one (compound 2);
[0058] Weigh 10.0 g of compound 1 and dissolve it in 30 mL of formamide, and react at 160° C. for 6 h under nitrogen protection. After the reaction is completed, the reaction solution is poured into ice water, filtered, and the filter cake is recrystallized with methanol to obtain compound 2.
[0059] 2) Preparation of 7-((4-aminophenyl)thio)quinazoline-4 by substitution reaction of 7-bromoquinazoline-4(3H)-one (compound 2) and p-aminothiophenol (compound 3) (3H)-ketone (compound 4);
[0060] Compound 2 2.0g, K 2 CO 3 1.26g was added to 10mL dimethyl sulfoxide, protected by argon, and the temperature was raised to 115℃; p-aminothiophenol 3 1.14g dimethyl sulfoxide (5mL) solution was added dropwise to the above reaction solution and reacted for 1h After the reaction, the reaction solution was poured into ice water, extracted with ethyl acetate, the o...
Embodiment 2
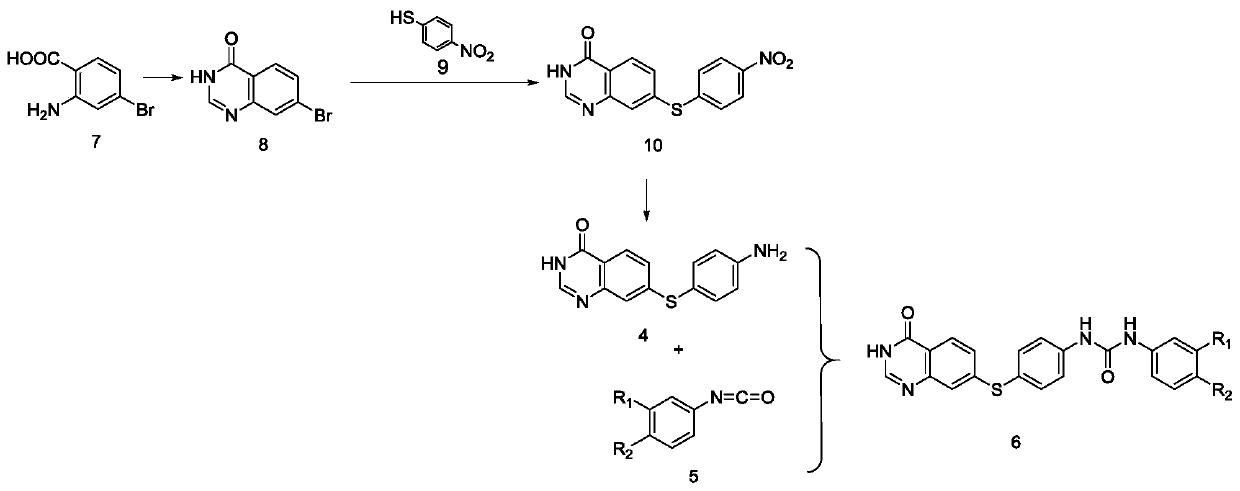
[0064] 1) 2-Amino-4-bromobenzoic acid (compound 7) and formamide are cyclized to prepare 7-bromoquinazolin-4(3H)-one (compound 8);
[0065] Use an analytical balance to weigh 5.1 g of compound 7 into a 250 mL round bottom flask, and add 100 mL of formamide solution, N 2 Microwave reaction under protection (150°C, 1.45h); after the reaction is over, ice water is added while hot, extracted with ethyl acetate, the organic phase is concentrated and left standing to precipitate a solid, which is suction filtered. The resulting filter cake is compound 8.
[0066] 2) 7-((4-nitrophenyl)thio)quinazoline-4 is prepared by reaction of 7-bromoquinazoline-4(3H)-one (compound 8) and p-nitrothiophenol (compound 9) (3H)-ketone (compound 10);
[0067] Compound 8 0.5g, p-nitrothiophenol 1.02g, K 2 CO 3 0.3g and 0.02g CuI were added to a 100mL round bottom flask. Under ice bath conditions, vacuum and nitrogen protection were applied. Then the temperature was raised to 130°C and reacted for 6h; after t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com