Patents
Literature
1058 results about "Suppressor cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Suppressor cells. Also found in: Dictionary, Thesaurus, Legal, Financial, Encyclopedia. cells of the immune system that inhibit or help to terminate an immune response, for example, suppressor macrophages and suppressor T cells.
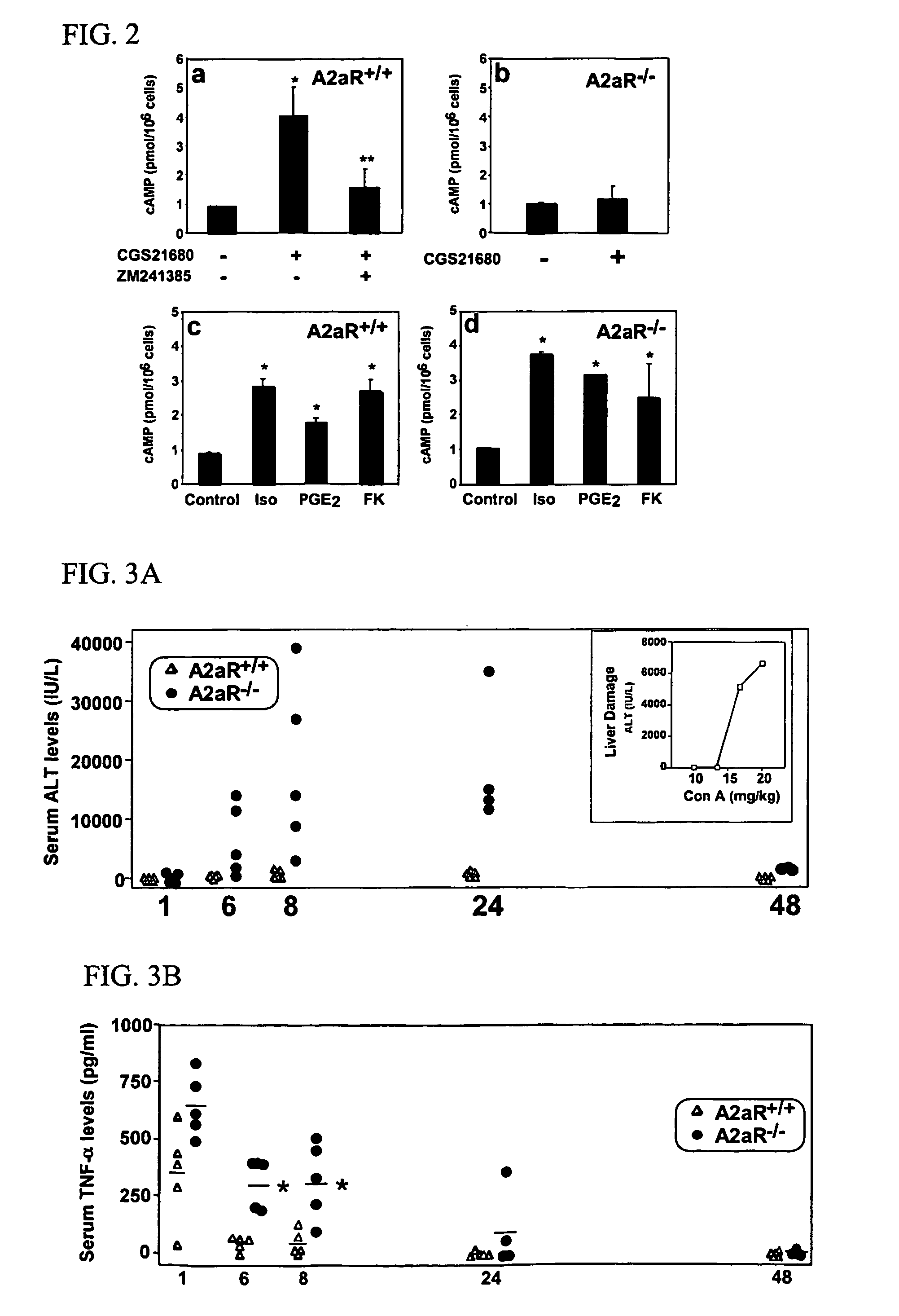
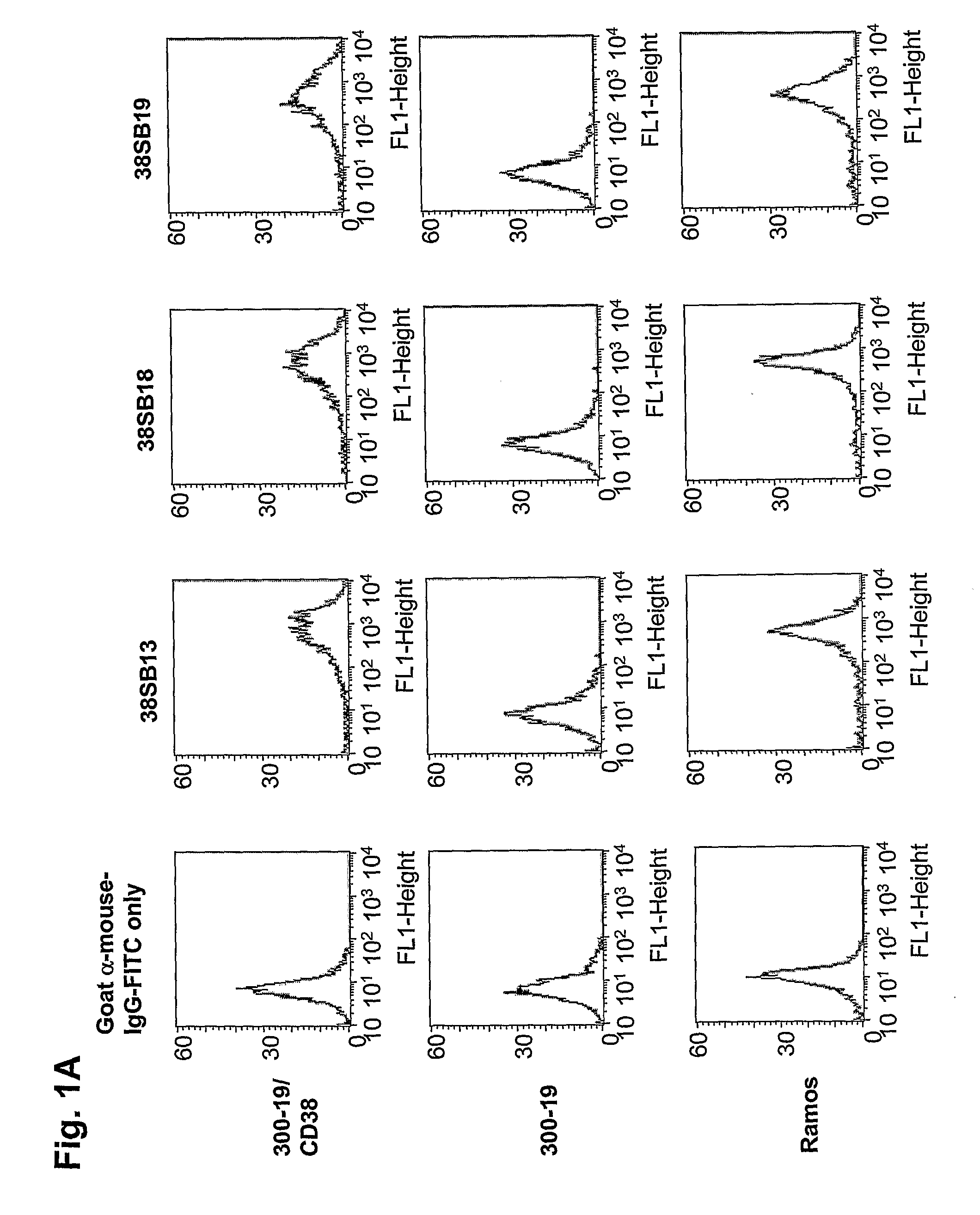
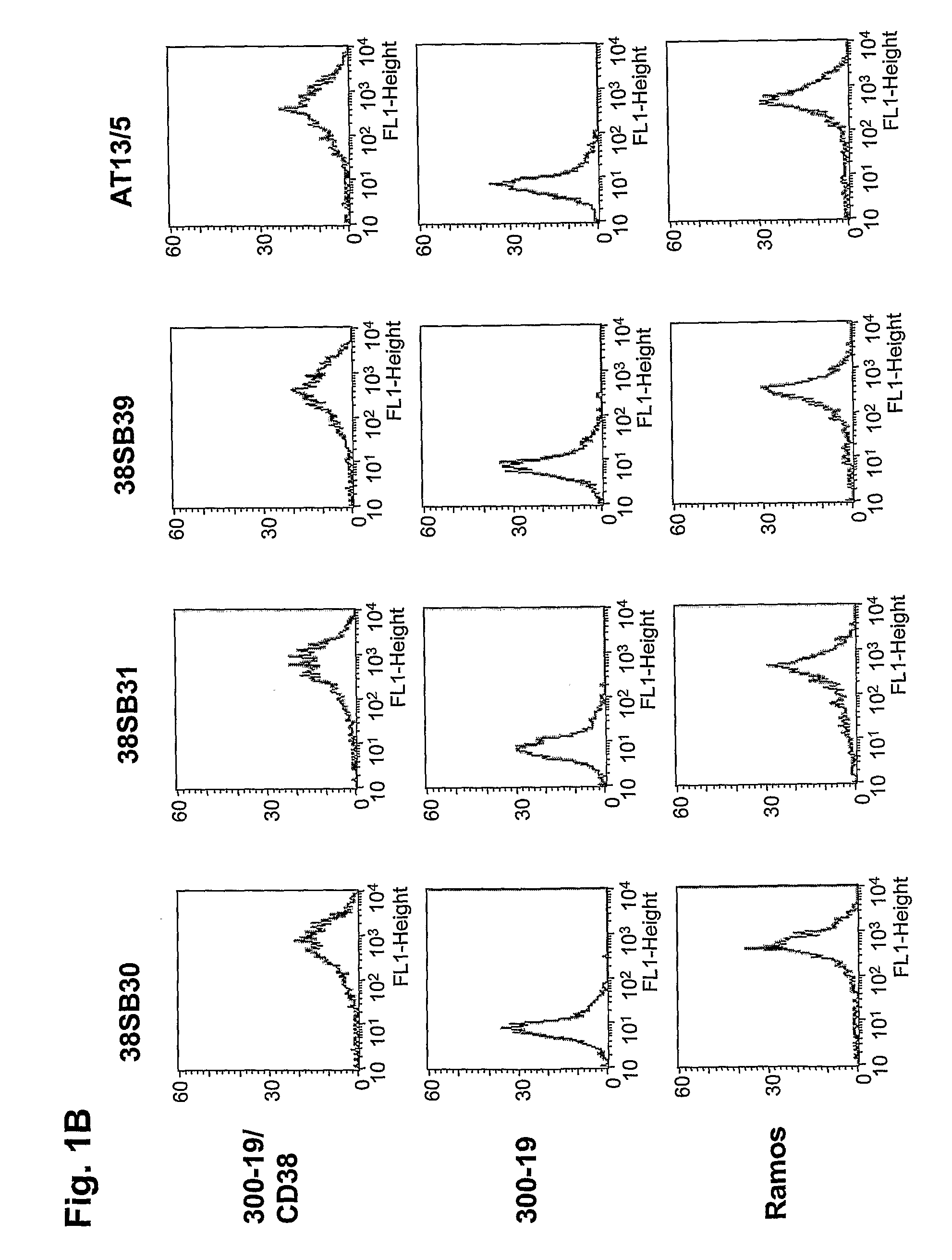
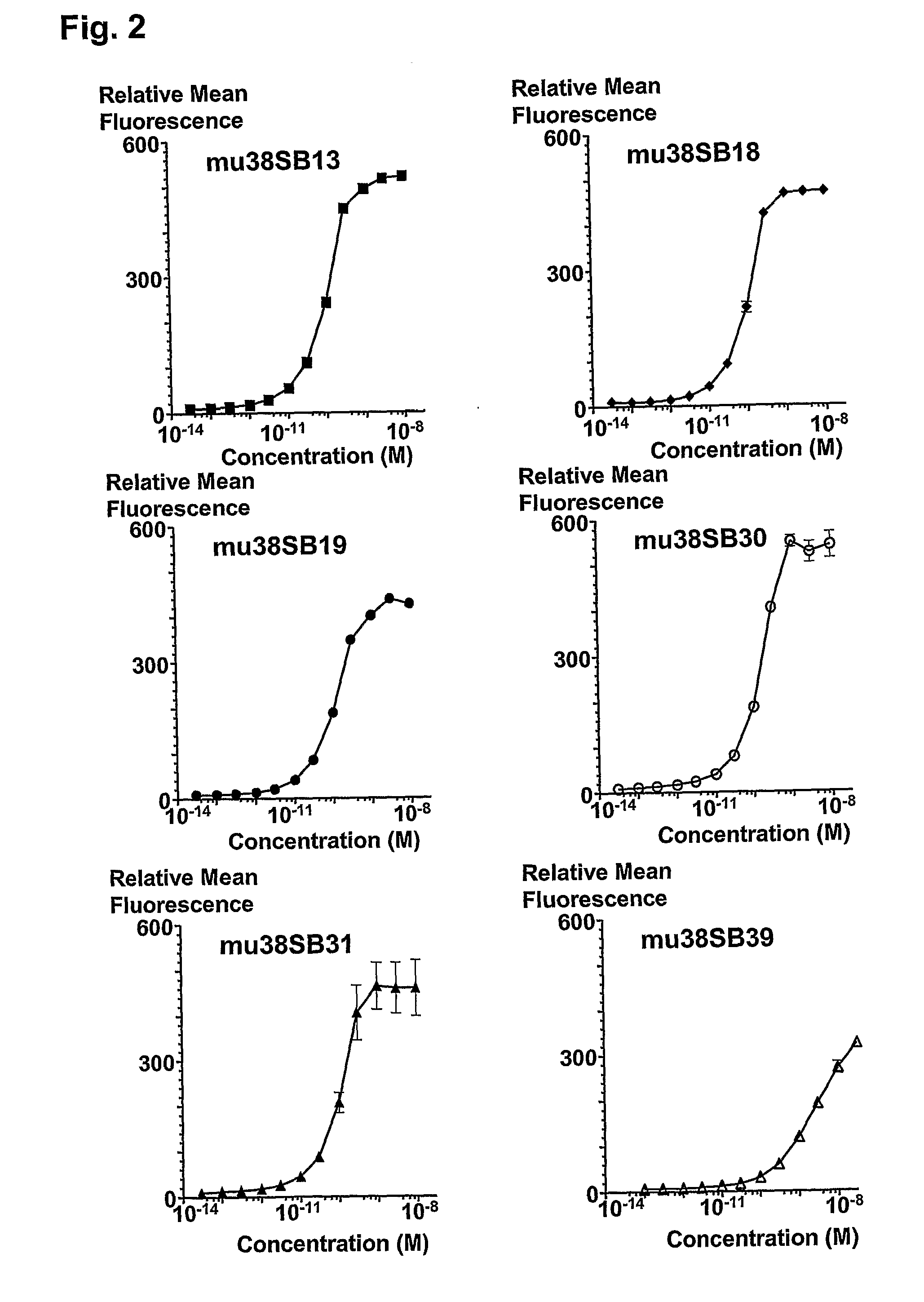
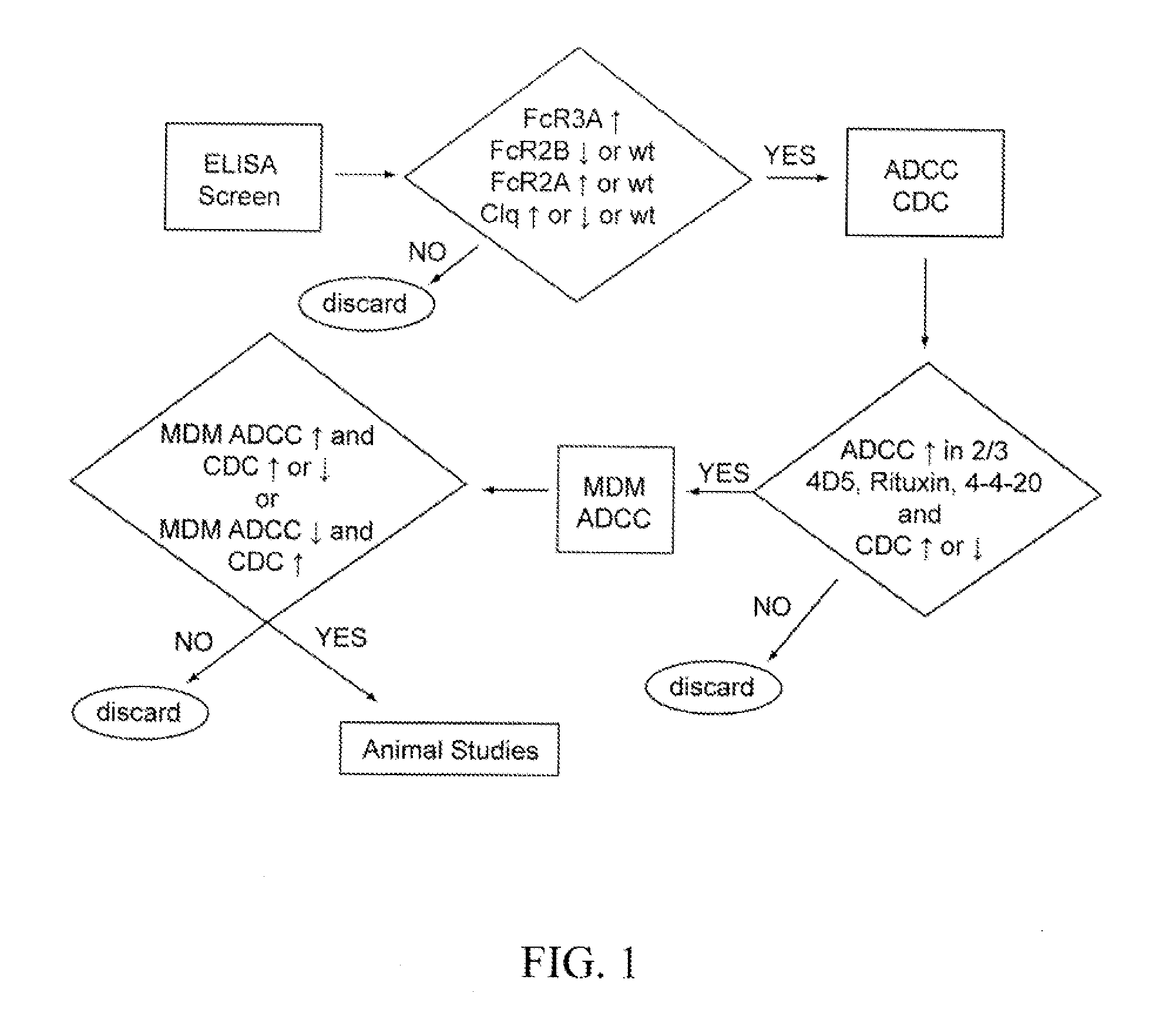
Novel Anti-cd38 antibodies for the treatment of cancer
ActiveUS20090304710A1Improve propertiesLess immunogenicSenses disorderAntipyreticComplement-dependent cytotoxicityAntibody fragments
Antibodies, humanized antibodies, resurfaced antibodies, antibody fragments, derivatized antibodies, and conjugates of same with cytotoxic agents, which specifically bind to CD38, are capable of killing CD38+ cells by apoptosis, antibody-dependent cell-mediated cytotoxicity (ADCC), and / or complement-dependent cytotoxicity (CDC). Said antibodies and fragments thereof may be used in the treatment of tumors that express CD38 protein, such as multiple myeloma, chronic lymphocytic leukemia, chronic myelogenous leukemia, acute myelogenous leukemia, or acute lymphocytic leukemia, or the treatment of autoimmune and inflammatory diseases such as systemic lupus, rheumatoid arthritis, multiple sclerosis, erythematosus, and asthma. Said derivatized antibodies may be used in the diagnosis and imaging of tumors that express elevated levels of CD38. Also provided are cytotoxic conjugates comprising a cell binding agent and a cytotoxic agent, therapeutic compositions comprising the conjugate, methods for using the conjugates in the inhibition of cell growth and the treatment of disease, and a kit comprising the cytotoxic conjugate. In particular, the cell binding agent is a monoclonal antibody, and epitope-binding fragments thereof, that recognizes and binds the CD38 protein.
Owner:SANOFI AVENTIS US LLC
Combined use of anti-cytokine antibodies or antagonists and anti-CD20 for treatment of B cell lymphoma
InactiveUS20020012665A1Avoiding and decreasing and resistanceOrganic active ingredientsIn-vivo radioactive preparationsFactor iiBiological activation
The present invention discloses combined therapies for treating hematologic malignancies, including B cell lymphomas and leukemias or solid non-hematologic tumors, comprising administration of anti-cytokine antibodies or antagonists to inhibit the activity of cytokines which play a role in perpetuating the activation of B cells. The administration of such antibodies and antagonists, particularly anti-IL10 antibodies and antagonists, is particularly useful for avoiding or decreasing the resistance of hematologic malignant cells or solid tumor cells to chemotherapeutic agents and anti-CD20 or anti-CD22 antibodies. The invention also provides combination therapies for solid tumors having B cell involvement comprising the administration of an anti-cytokine antibody and a B cell depleting antibody such as RITUXAN(R).
Owner:BIOGEN INC
Compositions and methods for inhibiting expression of Eg5 gene
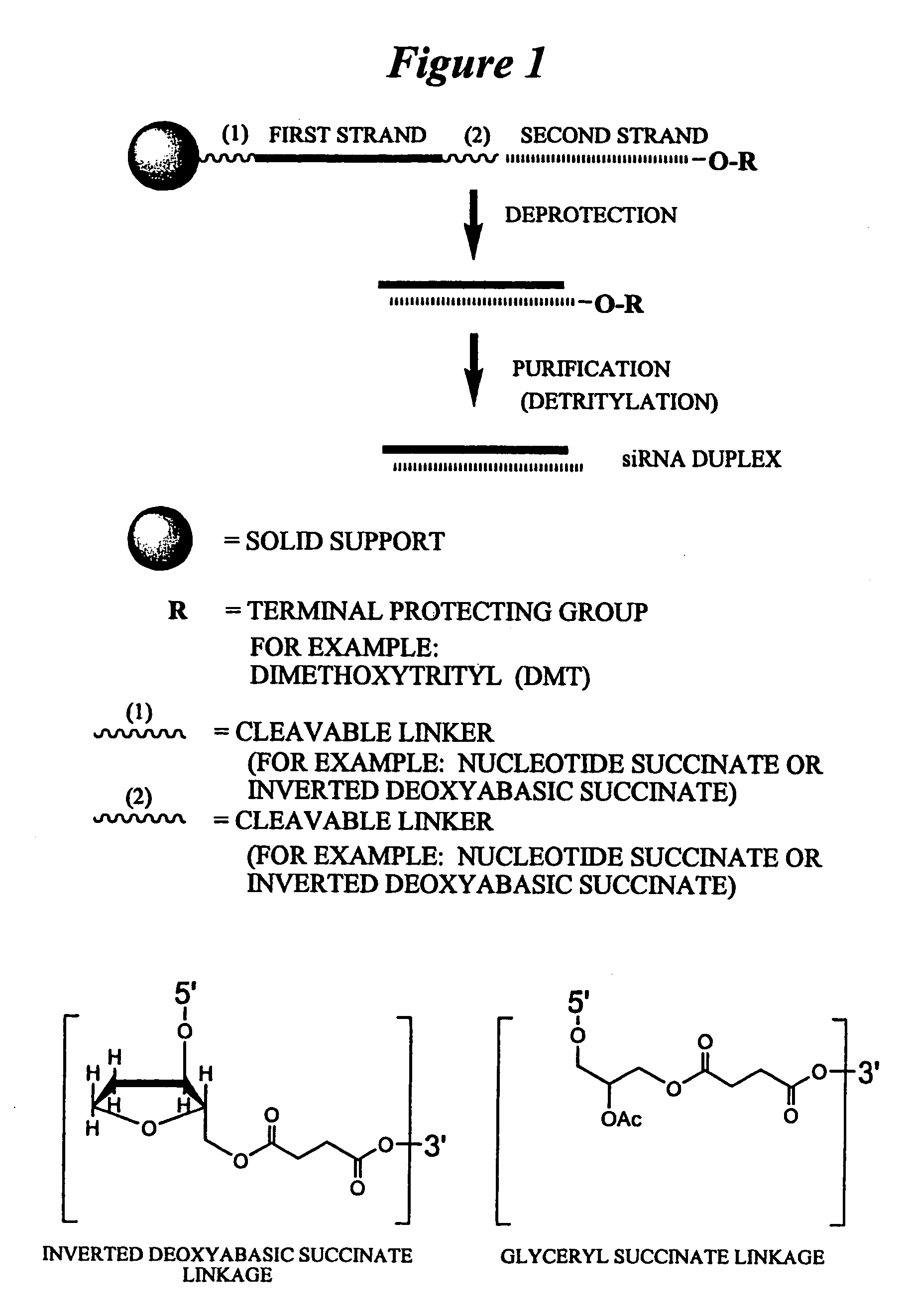
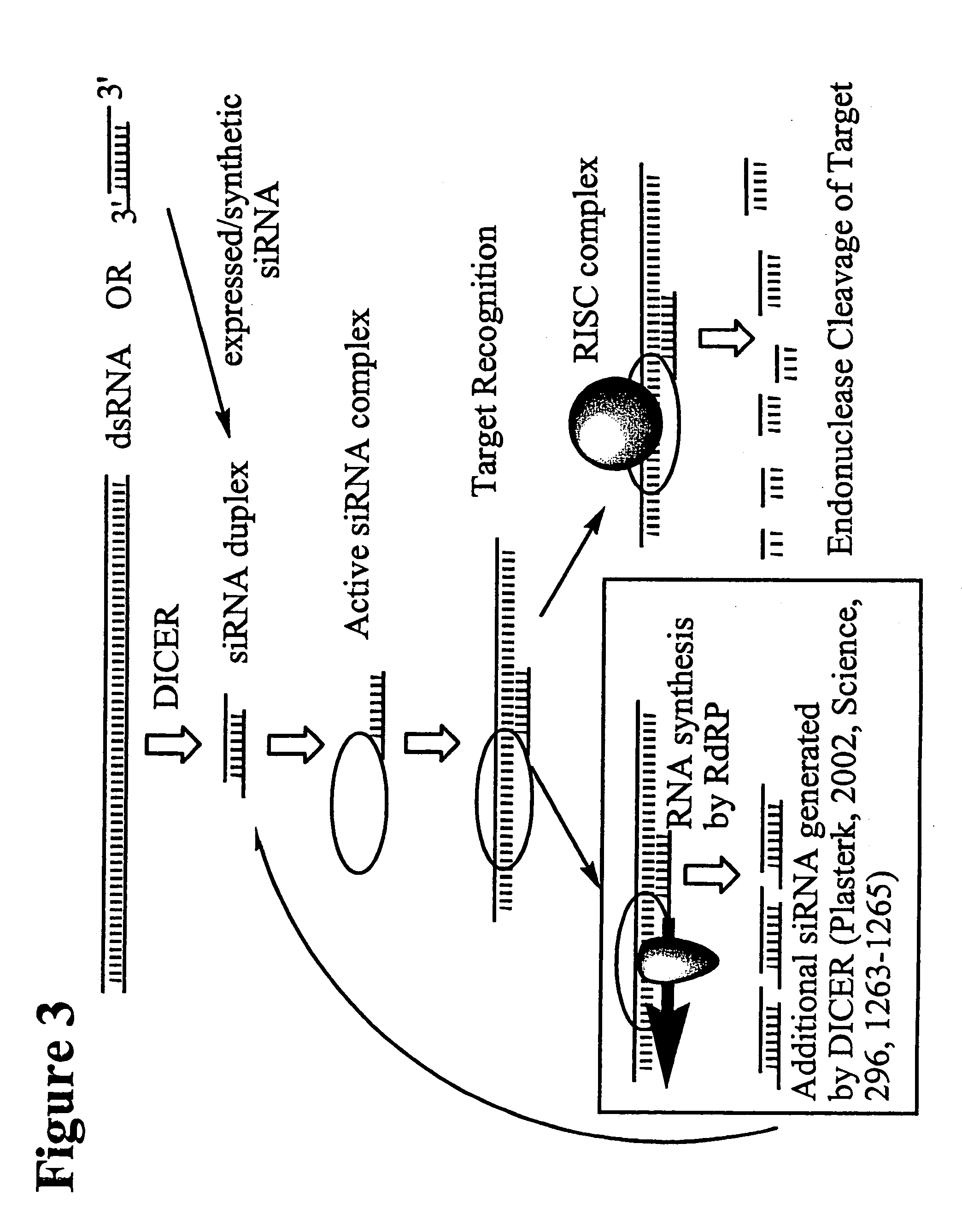
The invention relates to a double-stranded ribonucleic acid (dsRNA) for inhibiting the expression of the Eg5 gene (Eg5 gene), comprising an antisense strand having a nucleotide sequence which is less that 30 nucleotides in length, generally 19-25 nucleotides in length, and which is substantially complementary to at least a part of the Eg5 gene. The invention also relates to a pharmaceutical composition comprising the dsRNA together with a pharmaceutically acceptable carrier; methods for treating diseases caused by Eg5 expression and the expression of the Eg5 gene using the pharmaceutical composition; and methods for inhibiting the expression of the Eg5 gene in a cell.
Owner:ALNYLAM PHARMA INC
Pharmaceutical composition for suppression of apoptosis and method for delivering the same
InactiveUS20080132450A1Minimizing and avoiding systemic side effectEffectively suppress apoptosis and the development of diseasesAntibacterial agentsSenses disorderDiseaseMedicine
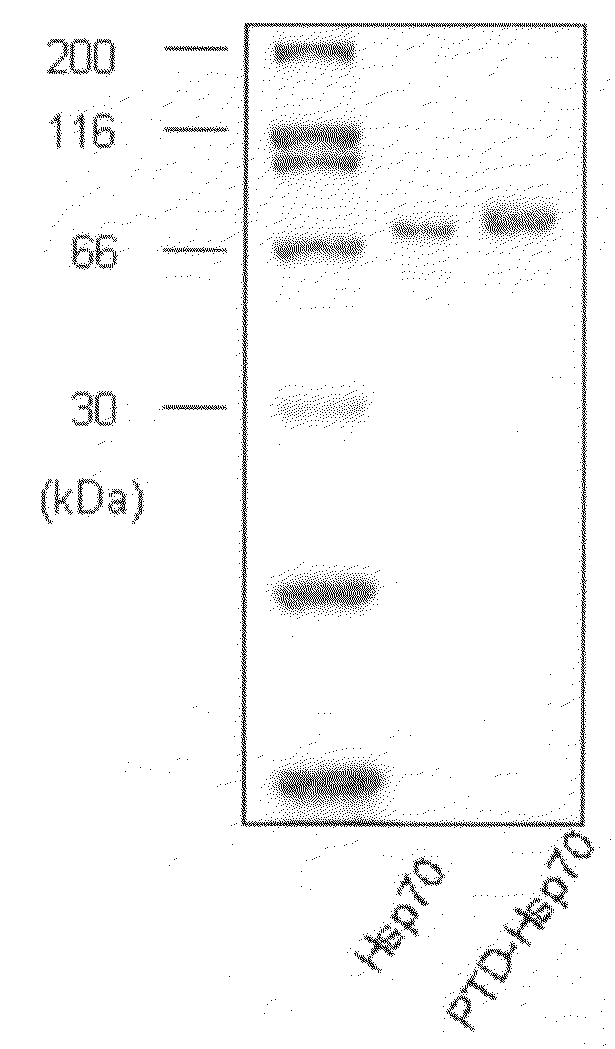
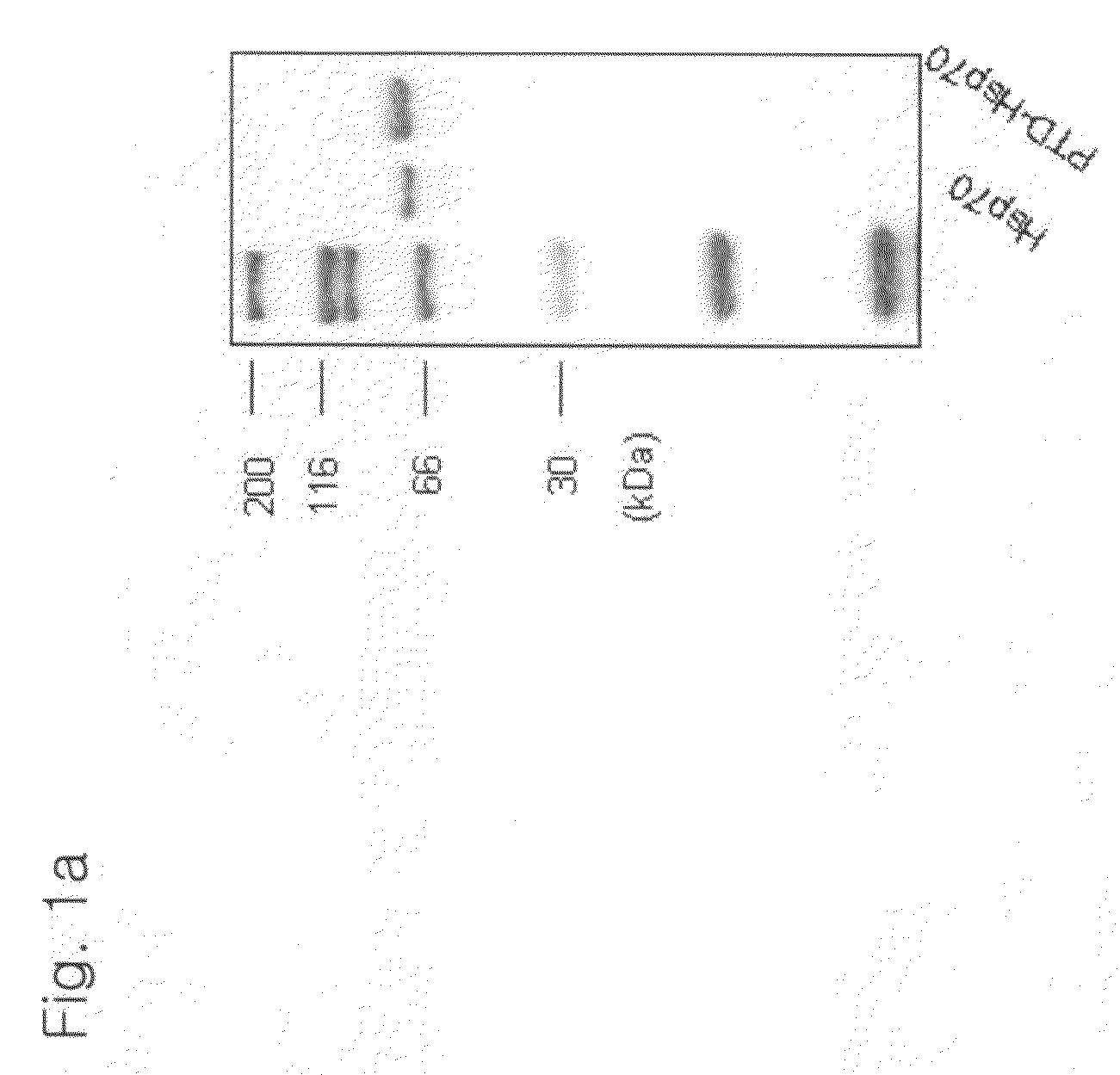
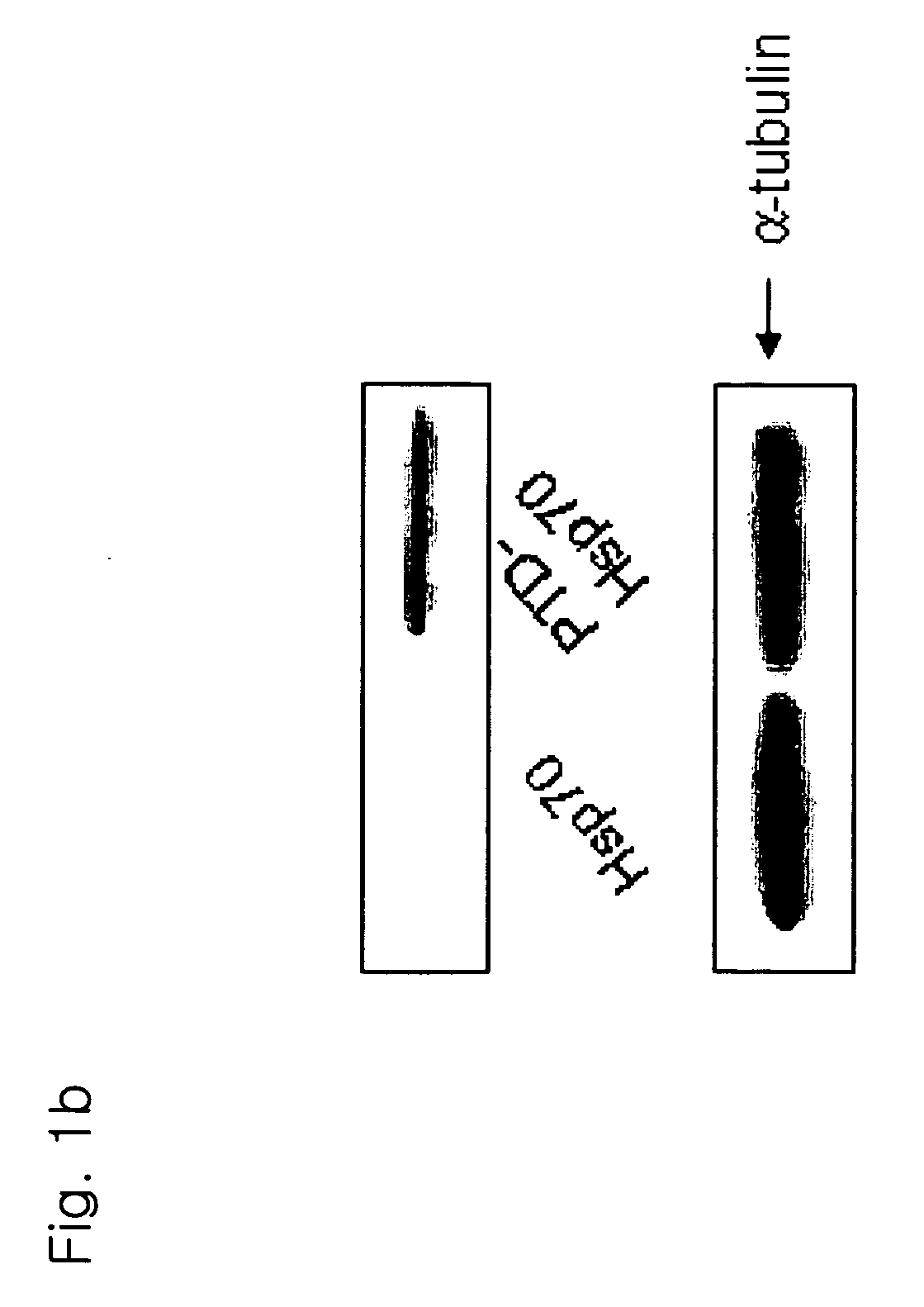
The present invention relates to a pharmaceutical composition for treating heart diseases, neurodegenerative diseases, and diseases and conditions caused by apoptosis, which contains a conjugate of a heat shock protein (Hsp) and a protein transduction domain (PTD). According to the present invention, PTD-Hsp70 effectively suppresses apoptosis under low-oxygen conditions.
Owner:FORHUMANTECH CO LTD
Ubiquitin ligase inhibitors
This invention describes compounds useful as ubiquitin ligase inhibitors. The compounds of the invention are useful as inhibitors of the biochemical pathways of organisms in which ubiquitination is involved. The invention also provides for pharmaceutical compositions comprising the compounds described in the invention for the treatment of conditions that require inhibition of ubiquitin ligases. Furthermore, the invention provides for methods of inhibiting ubiquitination in a cell comprising contacting a cell in which inhibition of ubiquitination is desired with a compound according to the invention.
Owner:RIGEL PHARMA
Novel use of aim 3 acting as a tumor suppressor
InactiveUS20060046250A1Prevent proliferationPromote phosphorylationCompound screeningApoptosis detectionApoptosisInducer Cells
The present invention relates to novel uses of AIM3 acting as a tumor suppressor, and more particularly to methods for using an AIM3 protein or a nucleic acid encoding the protein to activate ATM or ATR and to treat ATM- or ATR-mediated diseases. The AIM3 protein according to the present invention interacts directly with ATM / ATR so as to activate ATM / ATR and proteins regulated by ATM / ATR. Also, the AIM3 protein upregulates tumor suppressor gene p53 and its target genes so as to not only inhibit the proliferation of cells but also to induce apoptosis.
Owner:SCHWARZ HERBERT +1
Small molecule inhibitors of MDM2 and the uses thereof
InactiveUS7759383B2Extended half-lifeIncrease the function(s) of p53BiocideOrganic chemistrySensitized cellMdm2 Protein
Owner:RGT UNIV OF MICHIGAN
Compounds for enzyme inhibition
InactiveUS20070105786A1Inhibiting and reducing HIV infectionAffecting levelBiocideNervous disorderEnzyme inhibitionAziridine
Peptide-based compounds including heteroatom-containing, three-membered rings efficiently and selectively inhibit specific activities of N-terminal nucleophile (Ntn) hydrolases associated with the proteasome. The peptide-based compounds include an epoxide or aziridine, and functionalization at the N-terminus. Among other therapeutic utilities, the peptide-based compounds are expected to display anti-inflammatory properties and inhibition of cell proliferation. Oral administration of these peptide-based proteasome inhibitors is possible due to their bioavailability profiles
Owner:ONYX THERAPEUTICS
Cdki pathway inhibitors and uses thereof
ActiveUS20120071477A1Improve abilitiesGood curative effectBiocideOrganic active ingredientsChemistryDisease
The invention relates to the compounds and methods for inhibiting the Cyclin-Dependent Kinase Inhibitor (CDKI) pathway. More particularly, the invention relates to compounds and methods for inhibiting the CDKI pathway for studies of and intervention in senescence-related and other CDKI-related diseases.
Owner:SENEX BIOTECHNOLOGY INC
Methods for measuring cellular proliferation and destruction rates in vitro and in vivo
InactiveUS6461806B1Organic active ingredientsIn-vivo radioactive preparationsStable Isotope LabelingDisease
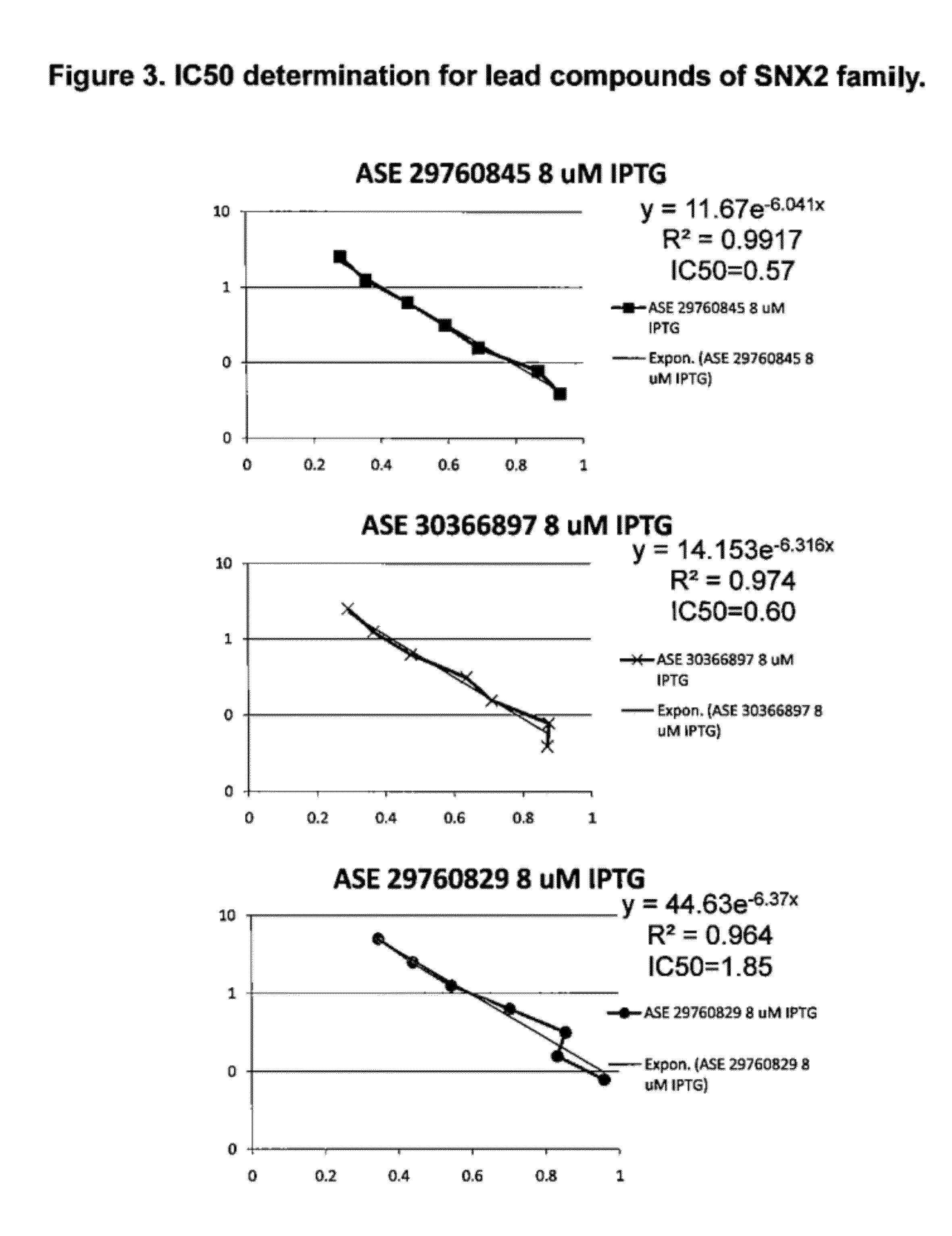
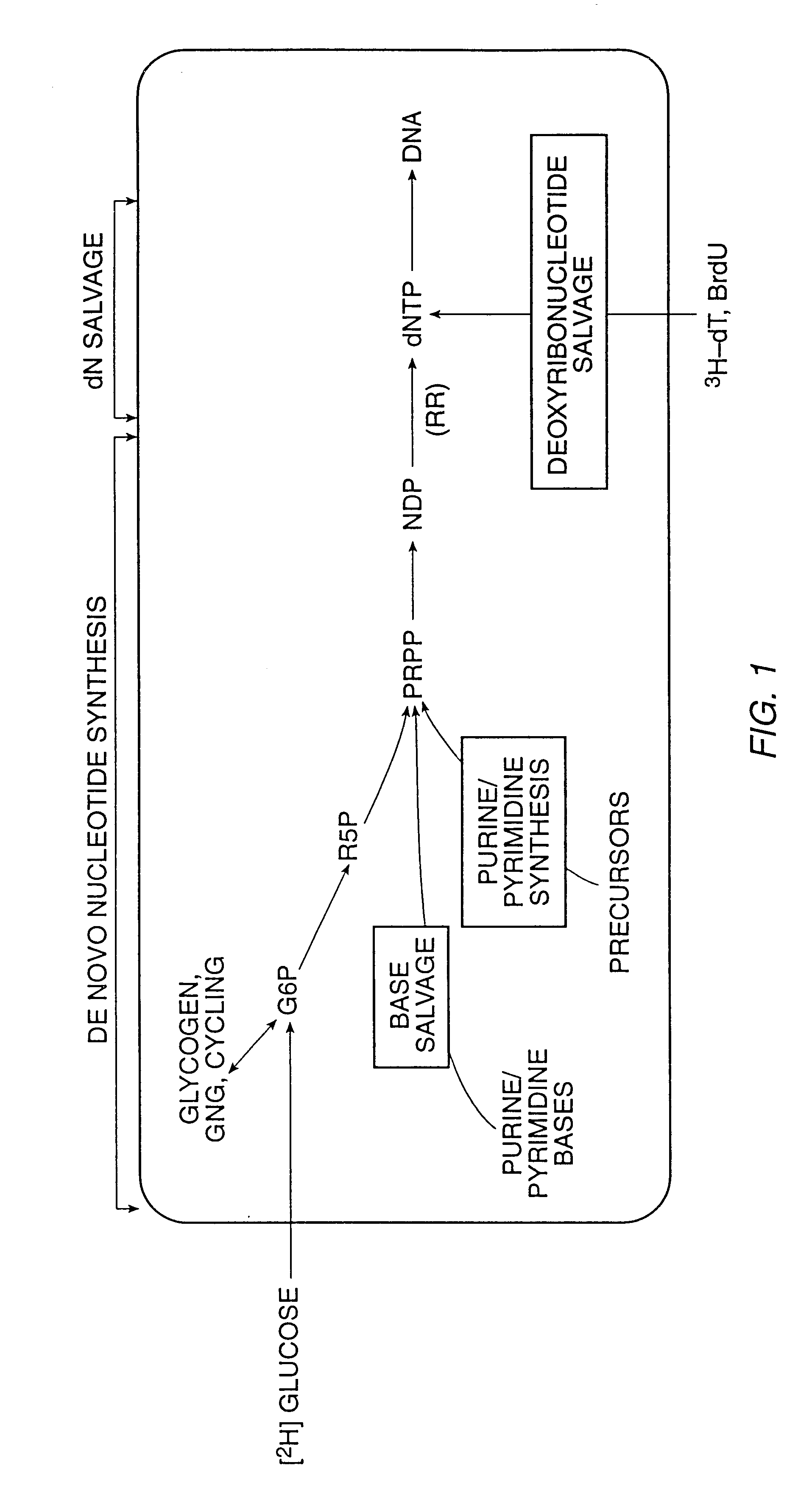
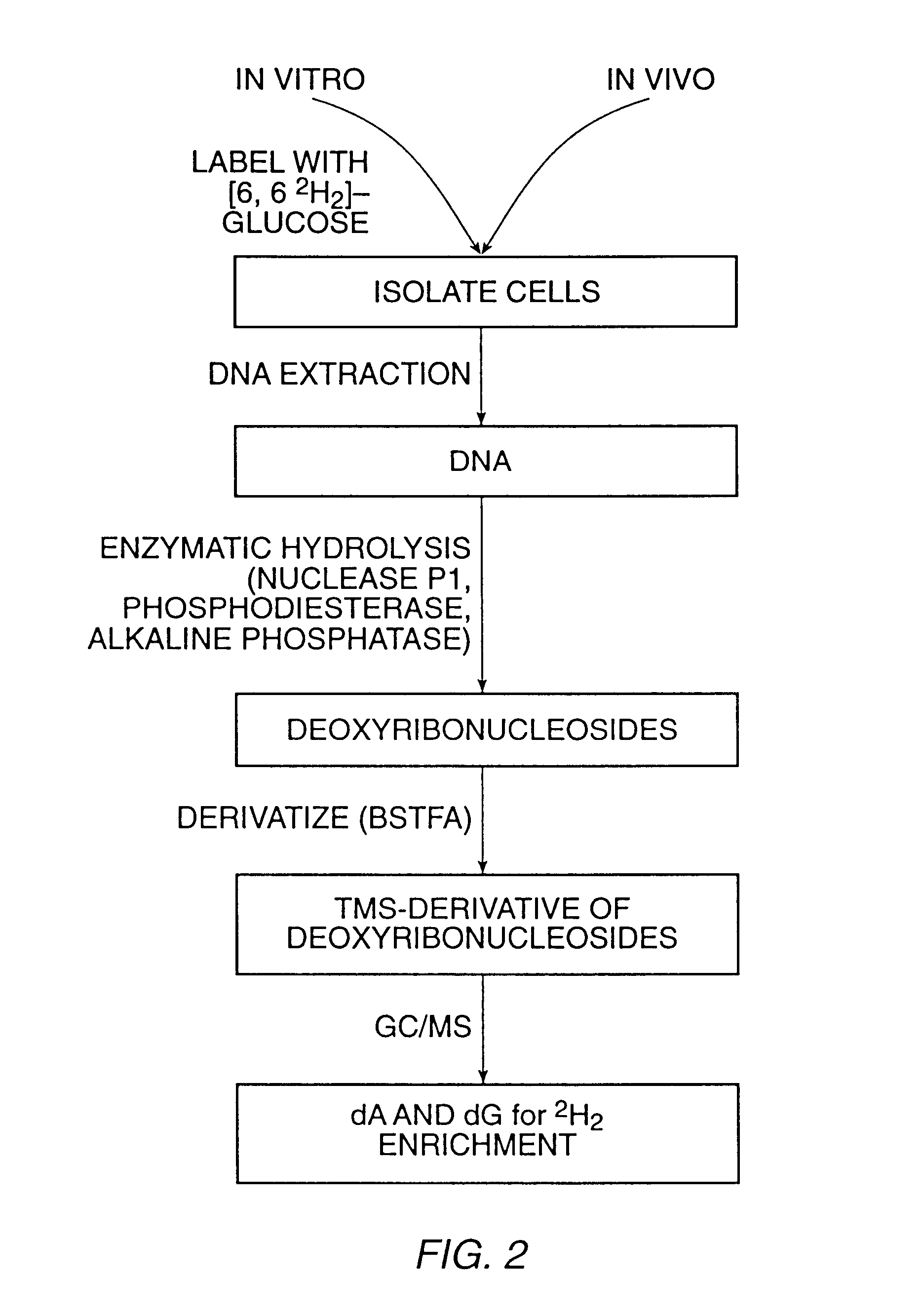
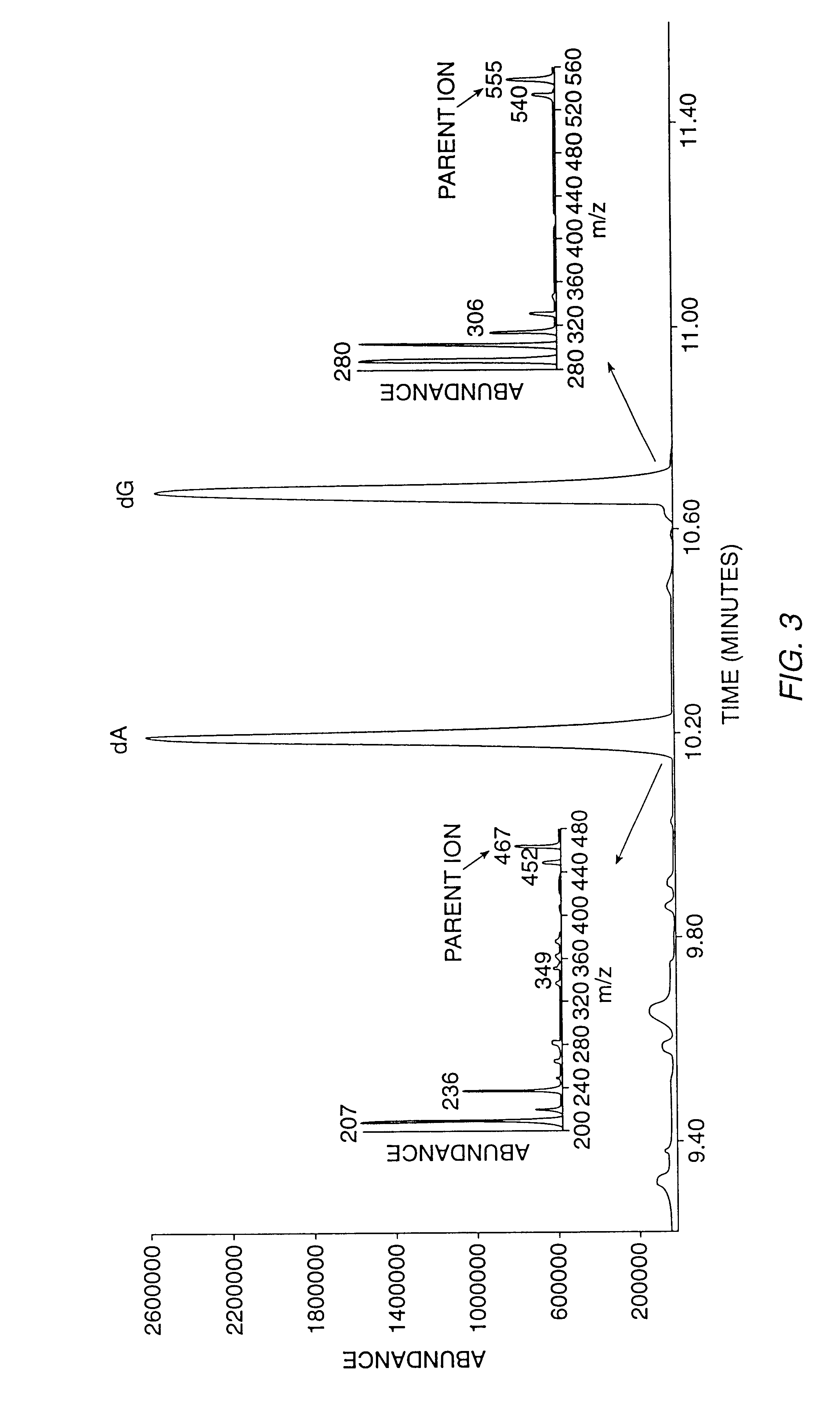
The present invention relates to methods for measuring the proliferation and destruction rates of cells by measuring deoxyribonucleic acid (DNA) synthesis and / or destruction. In particular, the methods utilize non-radioactive stable isotope labels to endogenously label DNA synthesized through the de novo nucleotide synthesis pathway in a cell. The amount of label incorporated in the DNA is measured as an indication of cellular proliferation. The decay of labeled DNA over time is measured as an indication of cellular destruction. Such methods do not involve radioactivity or potentially toxic metabolites, and are suitable for use both in vitro and in vivo. Therefore, the invention is useful for measuring cellular proliferation or cellular destruction rates in humans for the diagnosis, prevention, or management of a variety of disease conditions in which cellular proliferation or cellular destruction is involved. The invention also provides methods for measuring proliferation or destruction of T cells in a subject infected with human immunodeficiency virus (HIV) and methods of screening an agent for a capacity to induce or inhibit cellular proliferation or destruction. In addition, the invention provides methods for measuring cellular proliferation in a proliferating population which utilize both radioactive isotope labels and stable isotopes to endogenously label DNA through the de novo nucleotide synthesis pathway.
Owner:RGT UNIV OF CALIFORNIA
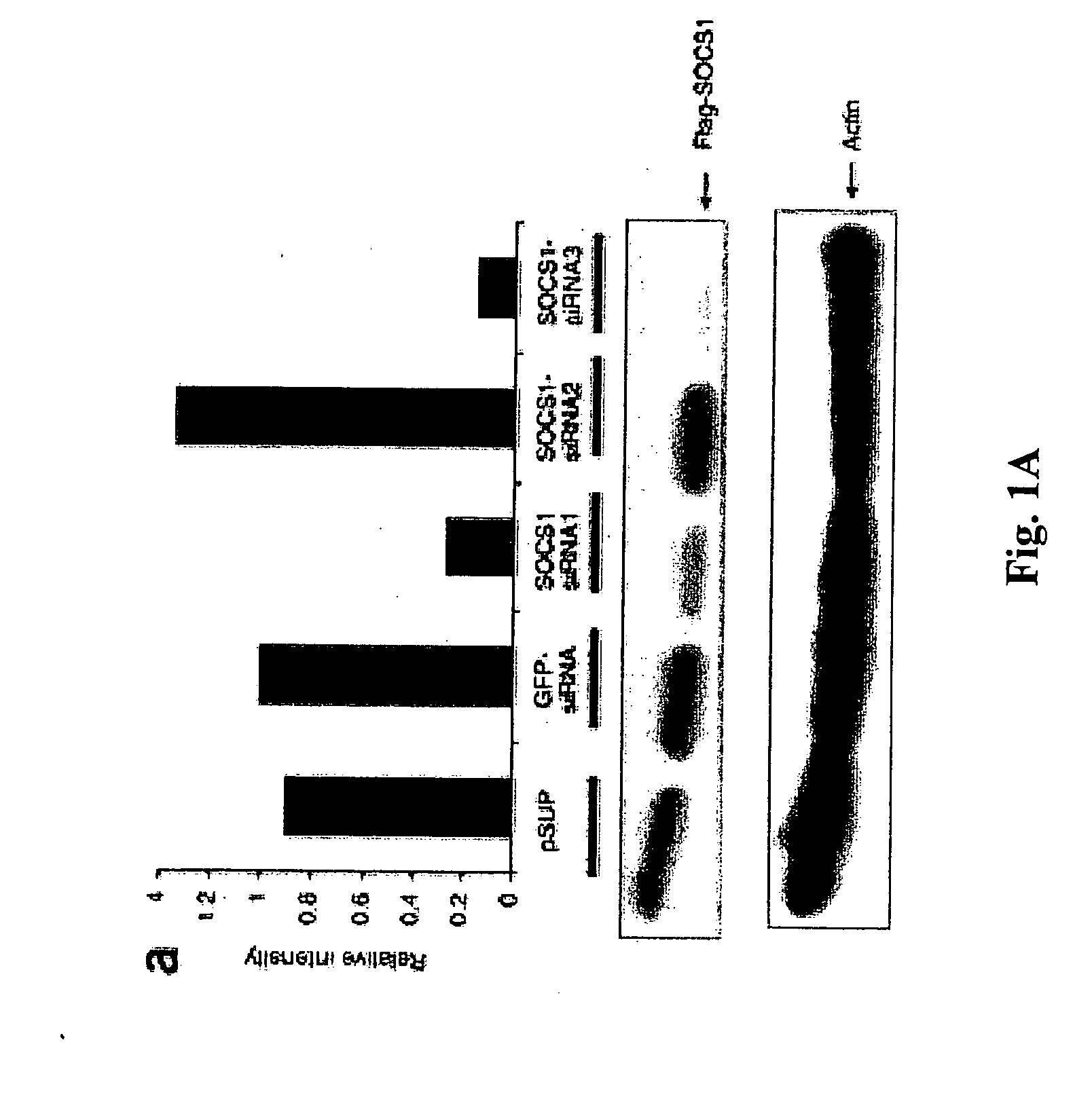
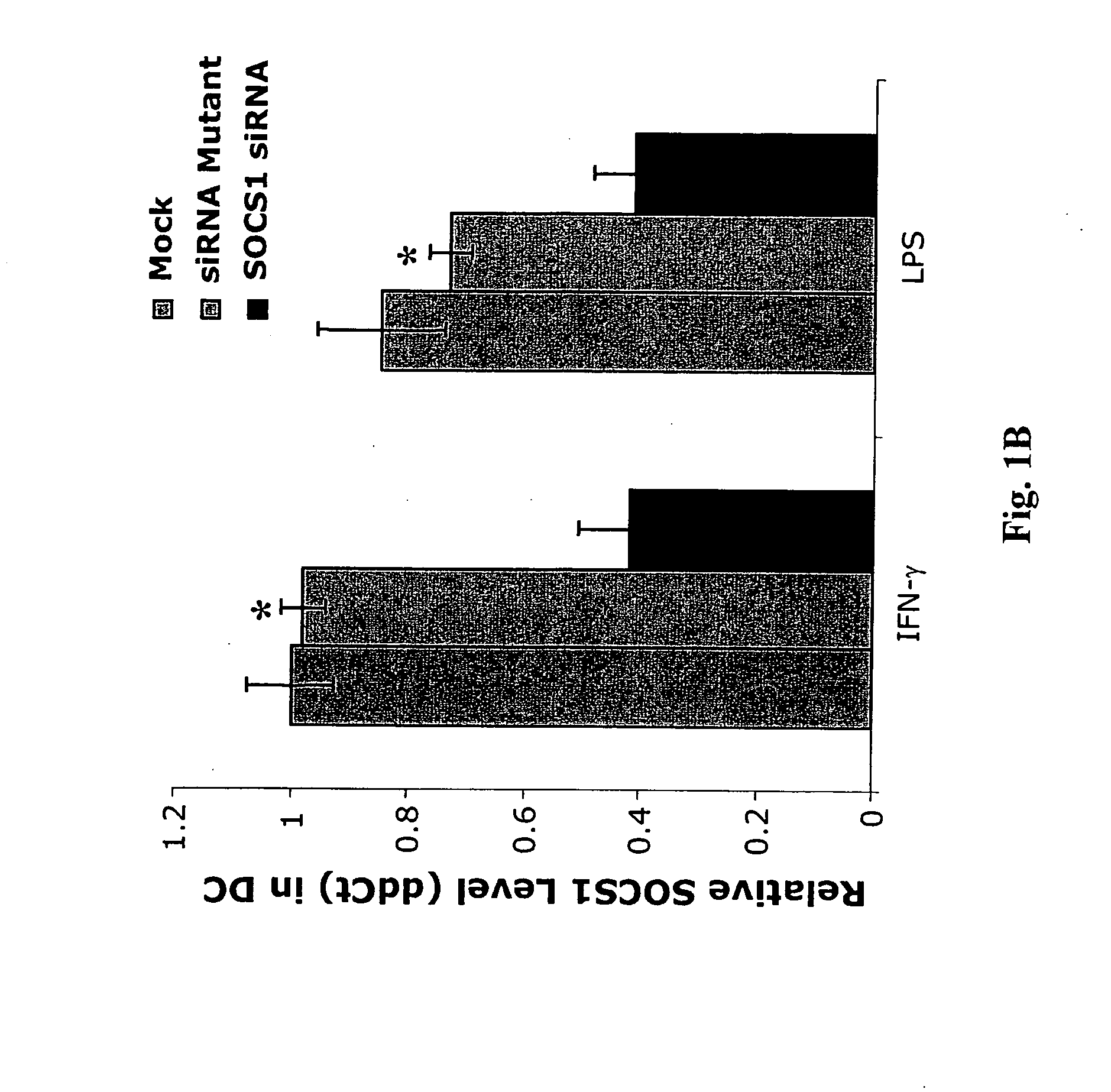
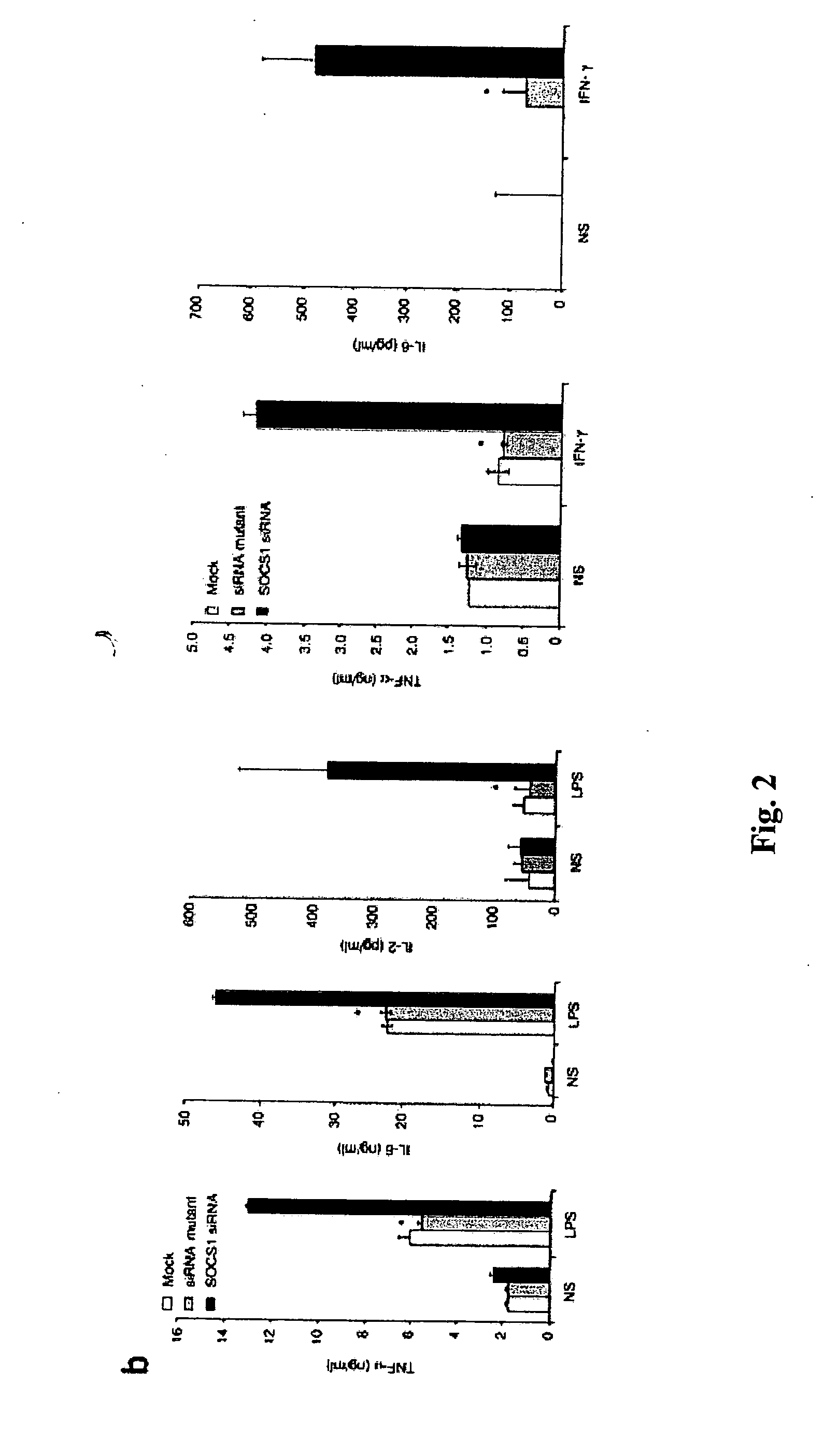
Modulation of negative immune regulators and applications for immunotherapy
ActiveUS20060292119A1Easy to integrateBiocideSsRNA viruses positive-senseVaccinationImmunocompetence
The invention includes compositions and methods for enhancing immunopotency of an immune cell by way of inhibiting a negative immune regulator in the cell. The present invention provides vaccines and therapies in which antigen presentation is enhanced through inhibition of negative immune regulators. The present invention also provides a mechanism to break self tolerance in tumor vaccination methods that rely on presentation of self tumor antigens.
Owner:BAYLOR COLLEGE OF MEDICINE
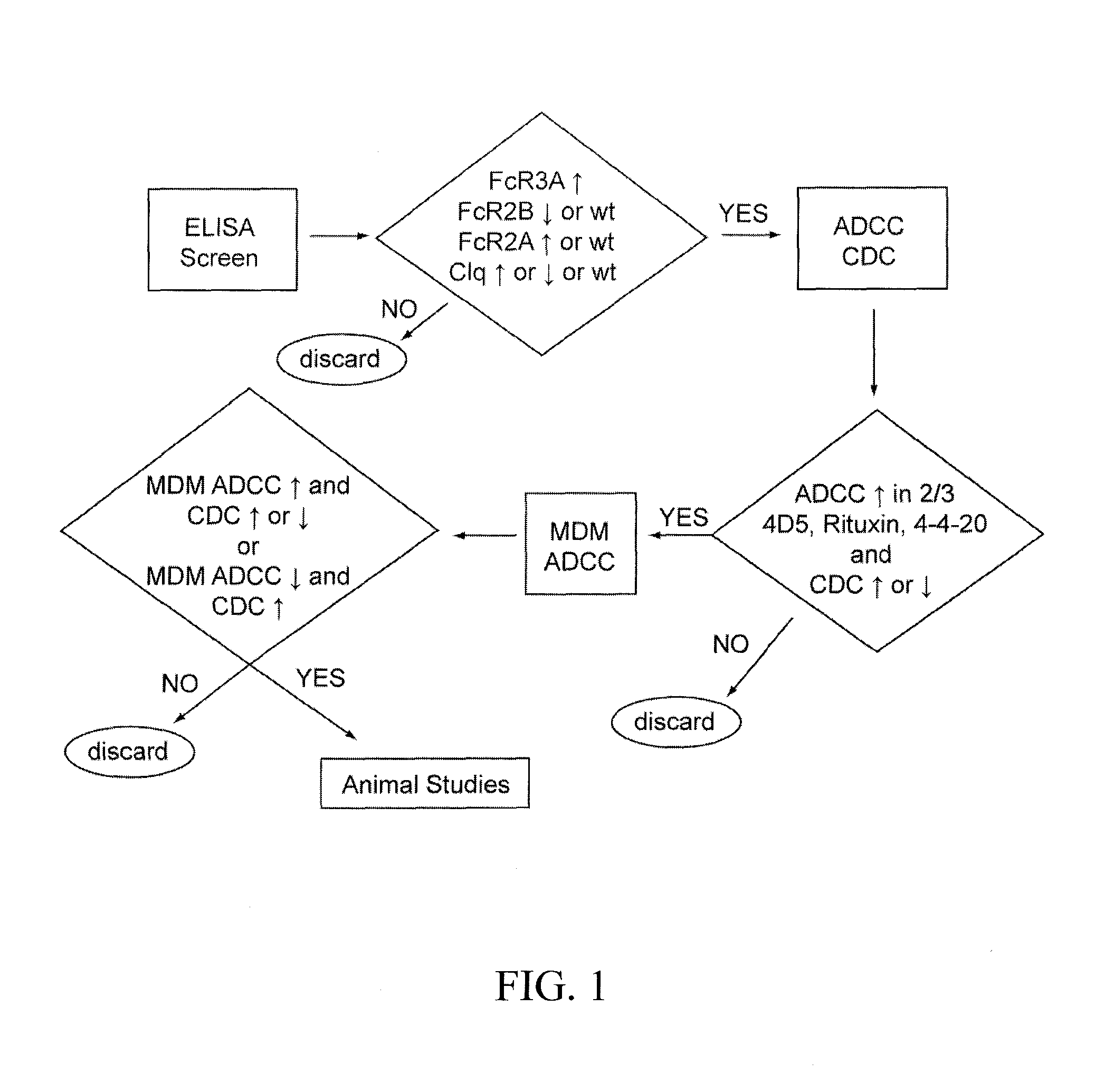
Methods for the treatment of disease using immunoglobulins having Fc regions with altered affinities for FcgammaRactivating and FcgammaRinhibiting
ActiveUS8652466B2Enhanced antibody effector functionGood curative effectImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsImmunologic disordersCancer prevention
The present invention relates to methods of treating or preventing cancer and other diseases using molecules, particularly polypeptides, more particularly immunoglobulins (e.g., antibodies), comprising a variant Fc region, wherein said variant Fc region comprises at least one amino acid modification relative to a wild-type Fc region, which variant Fc region binds an FcγR that activates a cellular effector (“FcγRActivating,” such as FcγRIIA or FcγRIIIA) and an FcγR that inhibits a cellular effector (“FcγRInhibiting,” such as FcγRIIA) with an altered Ratio of Affinities relative to the respective binding affinities of such FcγR for the Fc region of the wild-type immunoglobulin. The methods of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection where either an enhanced efficacy of effector cell function mediated by FcγR is desired (e.g., cancer, infectious disease) or an inhibited effector cell response mediated by FcγR is desired (e.g., inflammation, autoimmune disease).
Owner:MACROGENICS INC
Protein kinase modulators
InactiveUS20090239859A1Good treatment effectReduce cell proliferationBiocideNervous disorderFms-Like Tyrosine KinasePolymerase L
The invention relates in part to molecules having certain biological activities that include, but are not limited to, inhibiting cell proliferation, modulating protein kinase activity and modulating polymerase activity. Molecules of the invention can modulate Pim kinase activity and / or FMS-like tyrosine kinase (Flt) activity. The invention also relates in part to methods for using such molecules.
Owner:SENHWA BIOSCIENCES INC
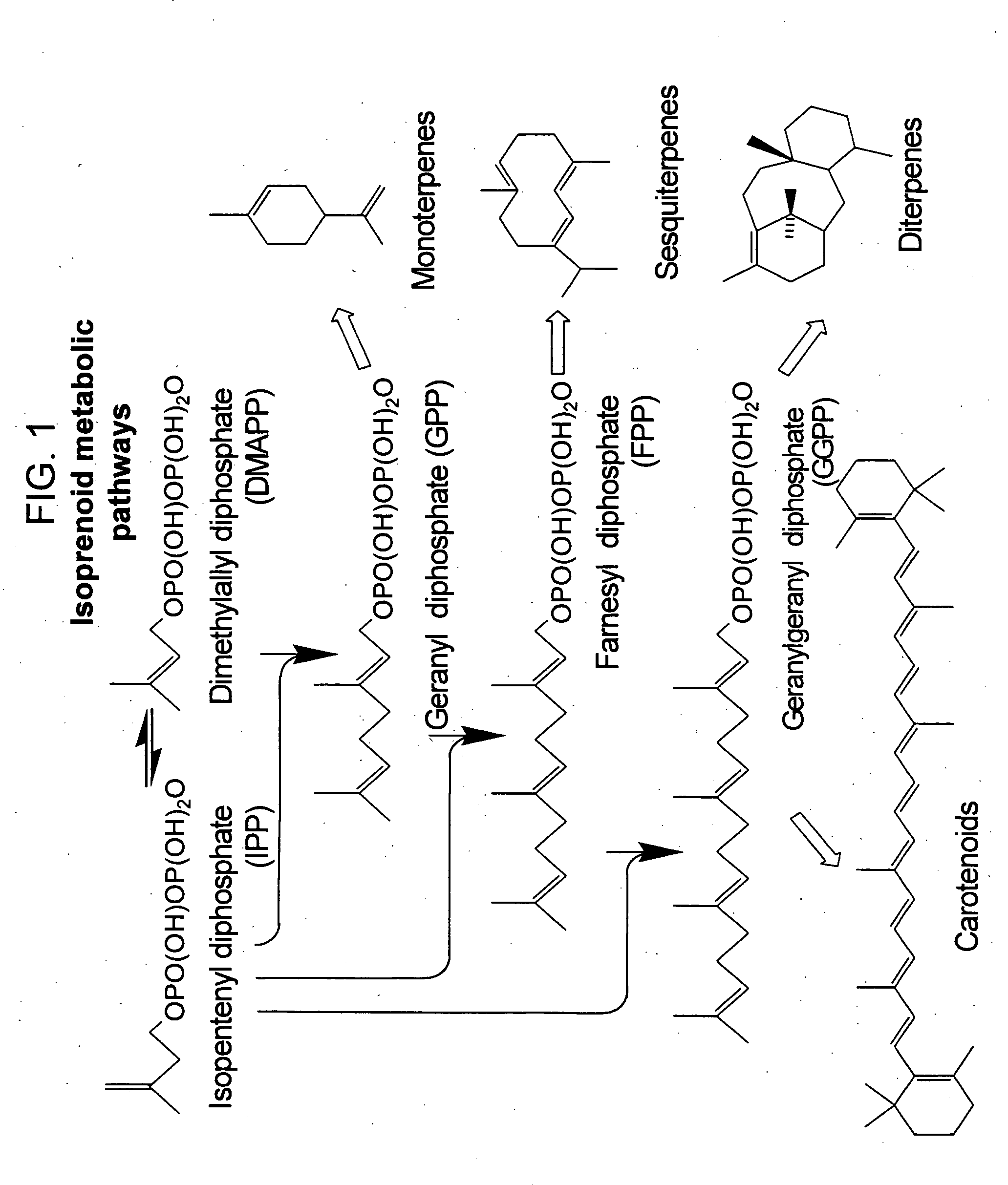
Method for enhancing production of isoprenoid compounds
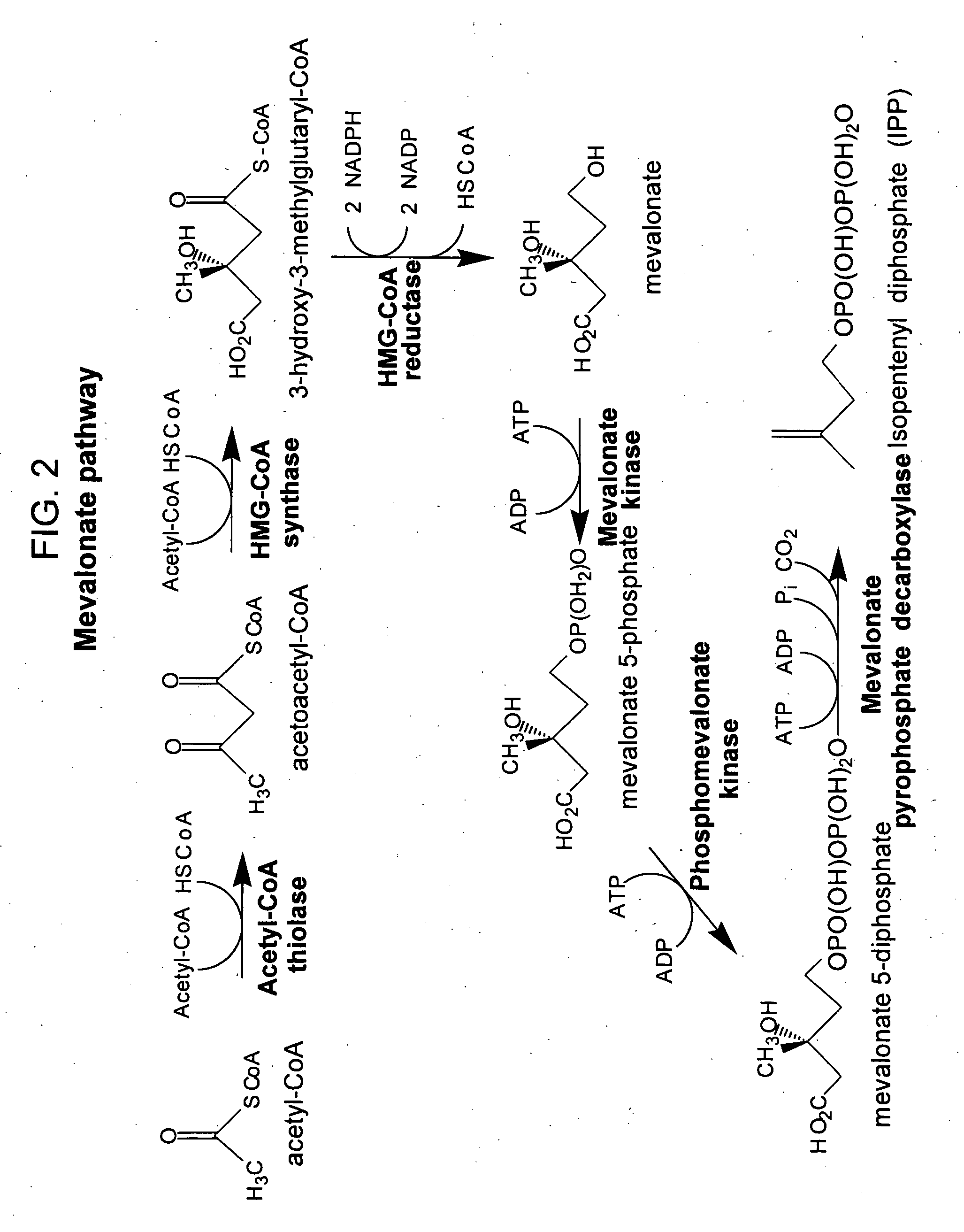
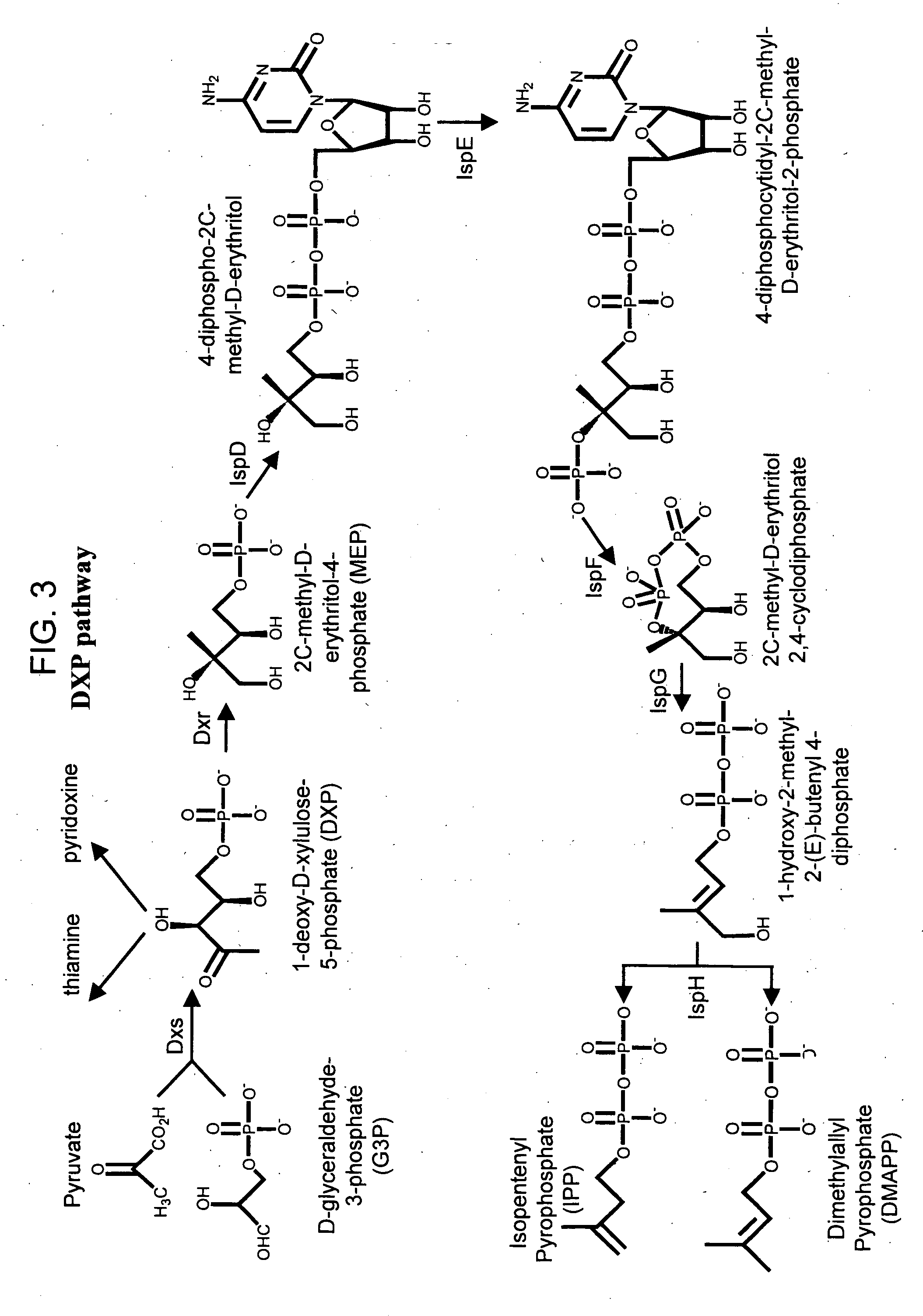
ActiveUS20060079476A1Modulating levelToxic levelGenetic material ingredientsOxidoreductasesENCODEMevalonate pathway
The present invention provides methods of producing an isoprenoid or an isoprenoid precursor in a genetically modified host cell. The methods generally involve modulating the level of hydroxymethylglutaryl-CoA (HMG-CoA) in the cell, such that the level of HMG-CoA is not toxic to the cell and / or does not substantially inhibit cell growth, but is maintained at a level that provides for high-level production of mevalonate, IPP, and other downstream products of an isoprenoid or isoprenoid pathway, e.g., polyprenyl diphosphates and isoprenoid compounds. The present invention further provides genetically modified host cells that are suitable for use in a subject method. The present invention further provides recombinant nucleic acid constructs for use in generating a subject genetically modified host cell, including recombinant nucleic acid constructs comprising nucleotide sequences encoding one or more mevalonate pathway enzymes, and recombinant vectors (e.g., recombinant expression vectors) comprising same. The present invention further provides methods for identifying nucleic acids that encode HMG-CoA reductase (HMGR) variants that provide for relief of HMG-CoA accumulation-induced toxicity. The present invention further provides methods for identifying agents that reduce intracellular accumulation of HMG-CoA.
Owner:RGT UNIV OF CALIFORNIA
Cyclic Inhibitors Of 11Beta-Hydroxysteroid Dehydrogenase 1
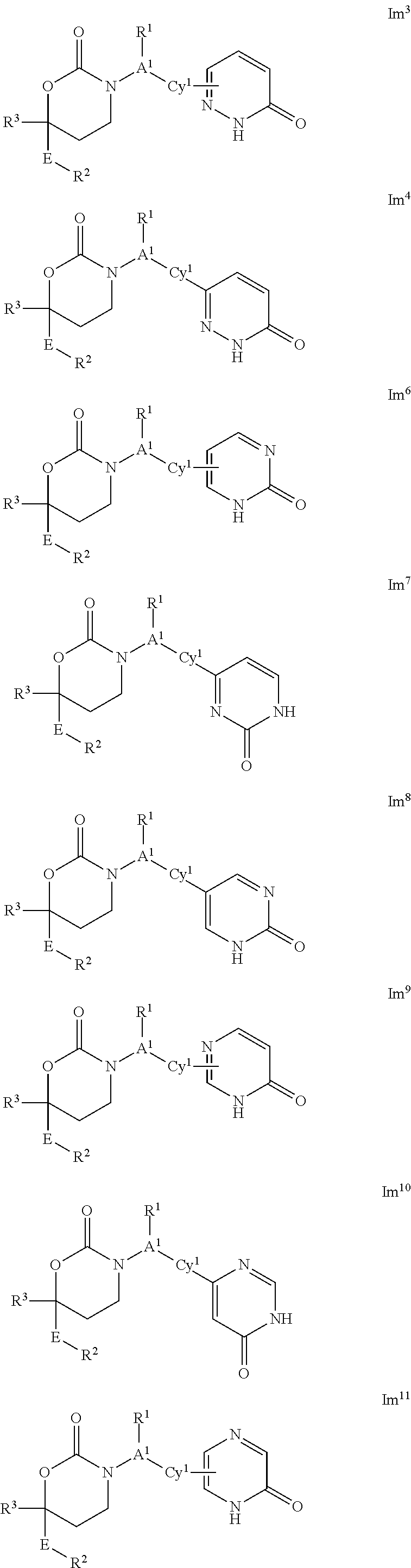
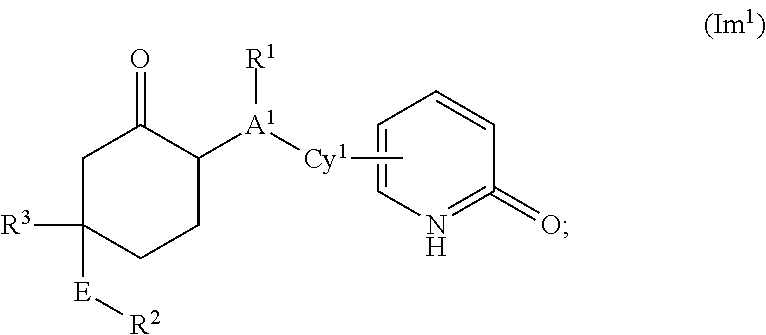
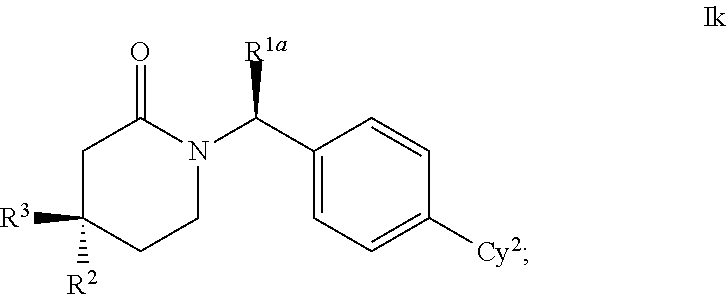
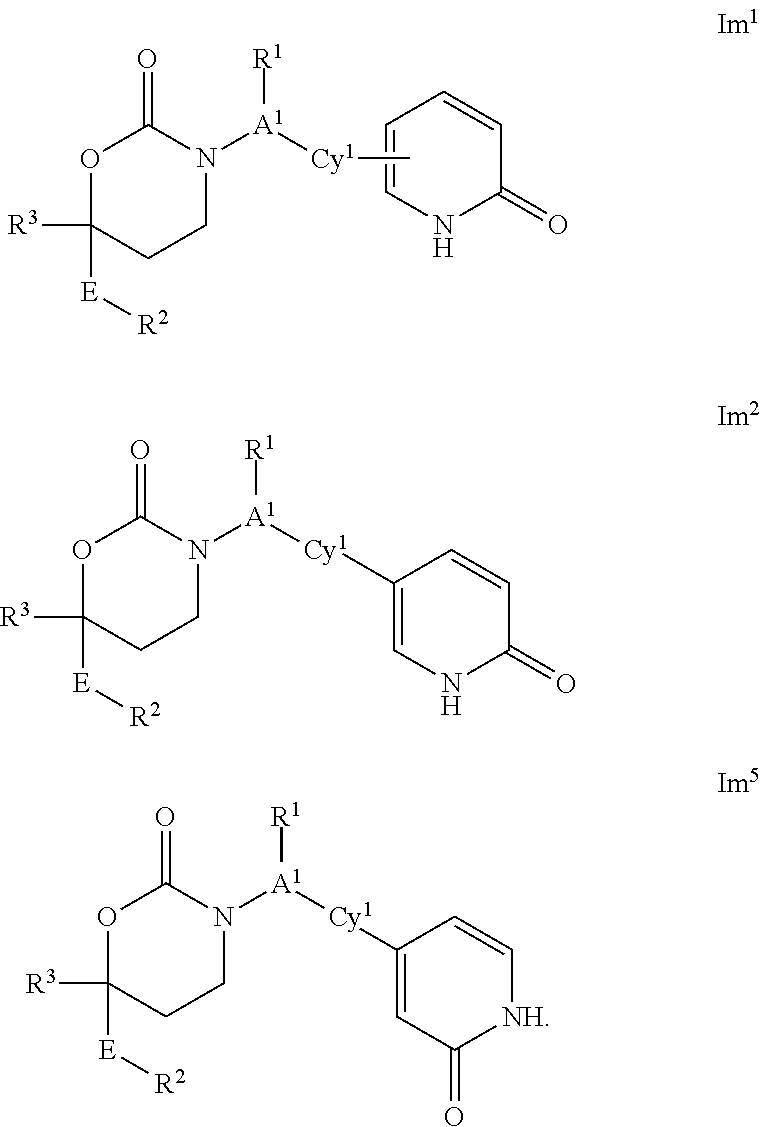
This invention relates to novel compounds of the Formula Il Ik, Im3, Im4, Im6-12, In3, In4, In6-12, lo3, lo4, lo6-12, Ip2, Ip4-7, pharmaceutically acceptable salts thereof, and pharmaceutical compositions thereof, which are useful for the therapeutic treatment of diseases associated with the modulation or inhibition of 11β-HSD1 in mammals. The invention further relates to pharmaceutical compositions of the novel compounds and methods for their use in the reduction or control of the production of Cortisol in a cell or the inhibition of the conversion of cortisone to Cortisol in a cell.
Owner:BOEHRINGER INGELHEIM INT GMBH +1
Cyclic Inhibitors of 11Beta-Hydroxysteroid Dehydrogenase 1
The invention relates to novel compounds of the Formula Ik, Im1, Im2, Im5, In1, In2, In5, Io1, Io2, Io5, Ip1, Ip3, pharmaceutically acceptable salts thereof, and pharmaceutical compositions thereof, which are useful for the therapeutic treatment of diseases associated with the modulation or inhibition of 11β-HSD1 in mammals. The invention further relates to pharmaceutical compositions of the novel compounds and methods for their use in the reduction or control of the production of Cortisol in a cell or the inhibition of the conversion of cortisone to cortisol in a cell.
Owner:BOEHRINGER INGELHEIM INT GMBH
RNA interference mediated inhibition of intercellular adhesion molecule (ICAM) gene expression using short interfering nucleic acid (siNA)
InactiveUS20050187174A1Improve bioavailabilityMinimize the possibilityCompounds screening/testingSpecial deliveryCompound (substance)Regulator gene
This invention relates to compounds, compositions, and methods useful for modulating intercellular adhesion molecule (ICAM) gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of ICAM gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of ICAM genes.
Owner:SIRNA THERAPEUTICS INC
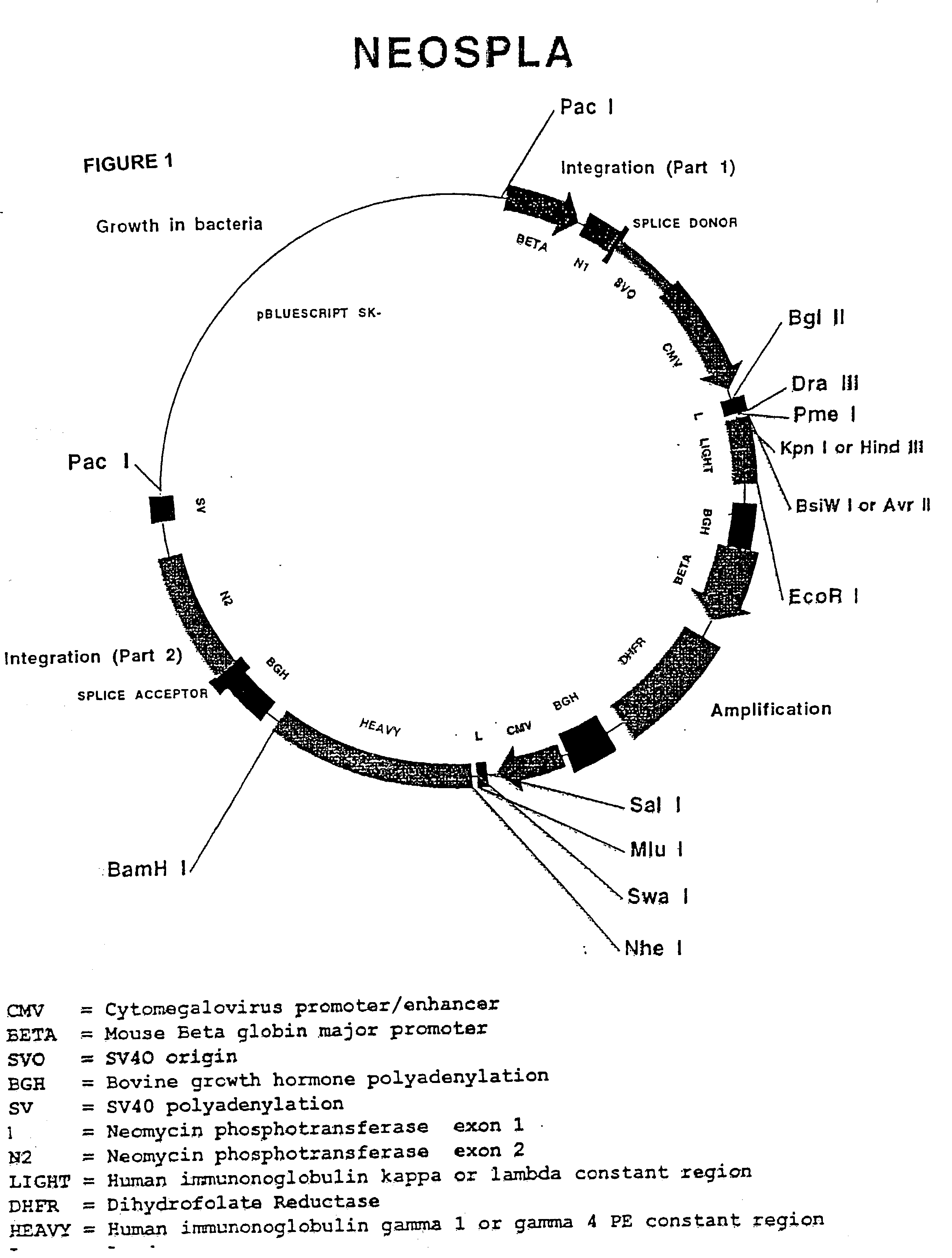
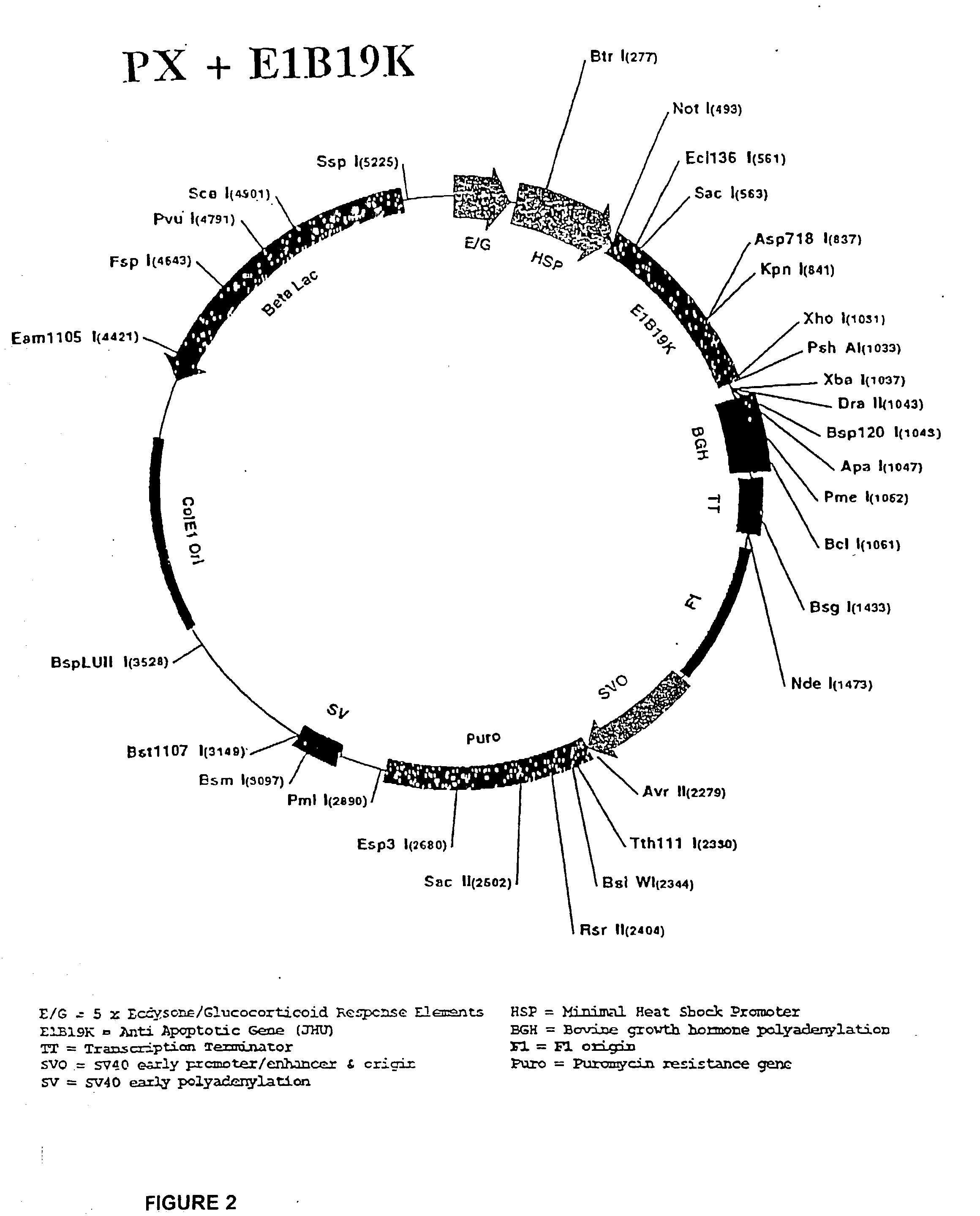
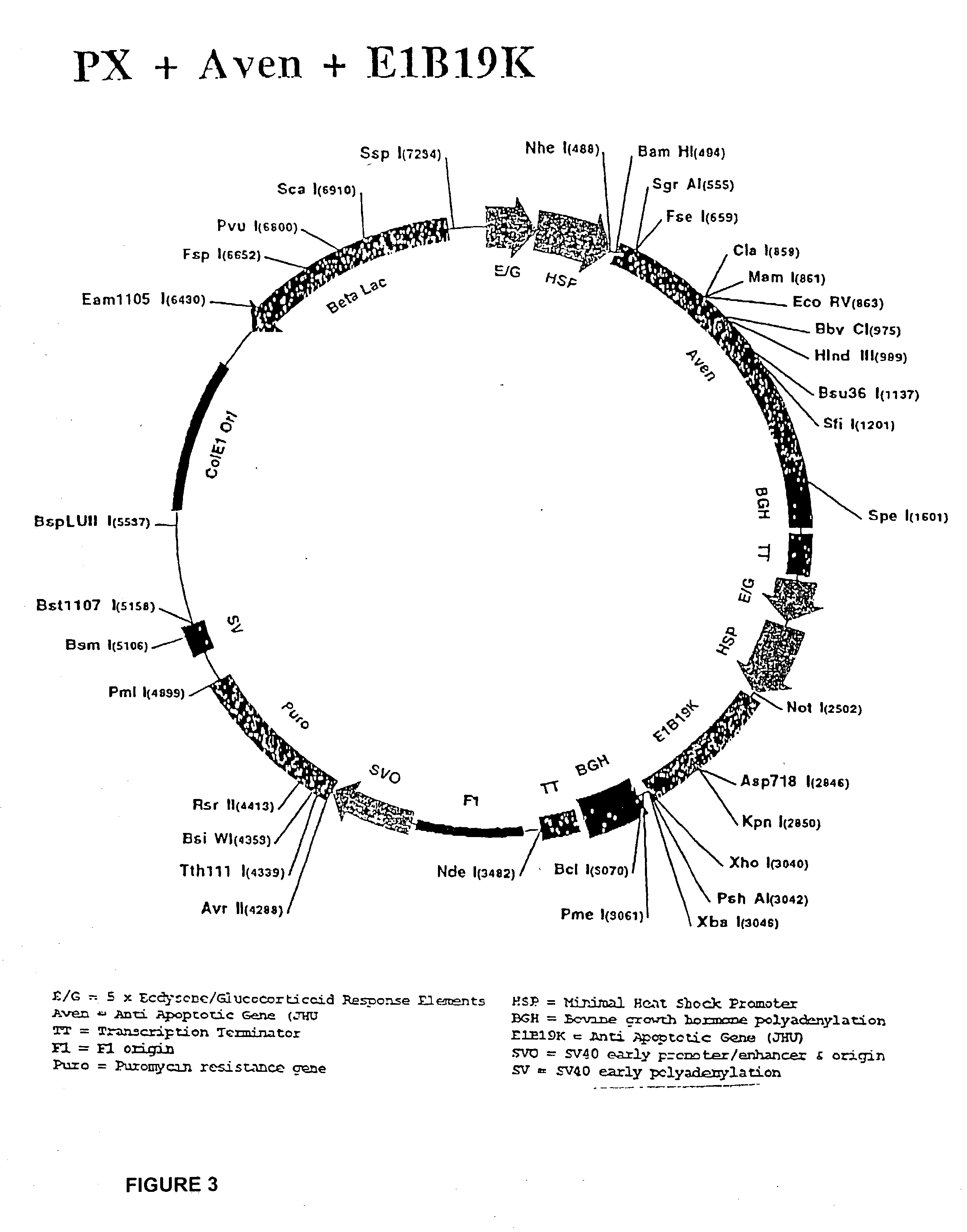
Molecular vaccines employing nucleic acid encoding anti-apoptotic proteins
InactiveUS20070026076A1Increase the number ofEasy to demonstratePowder deliveryVirusesDendritic cellVaccine Potency
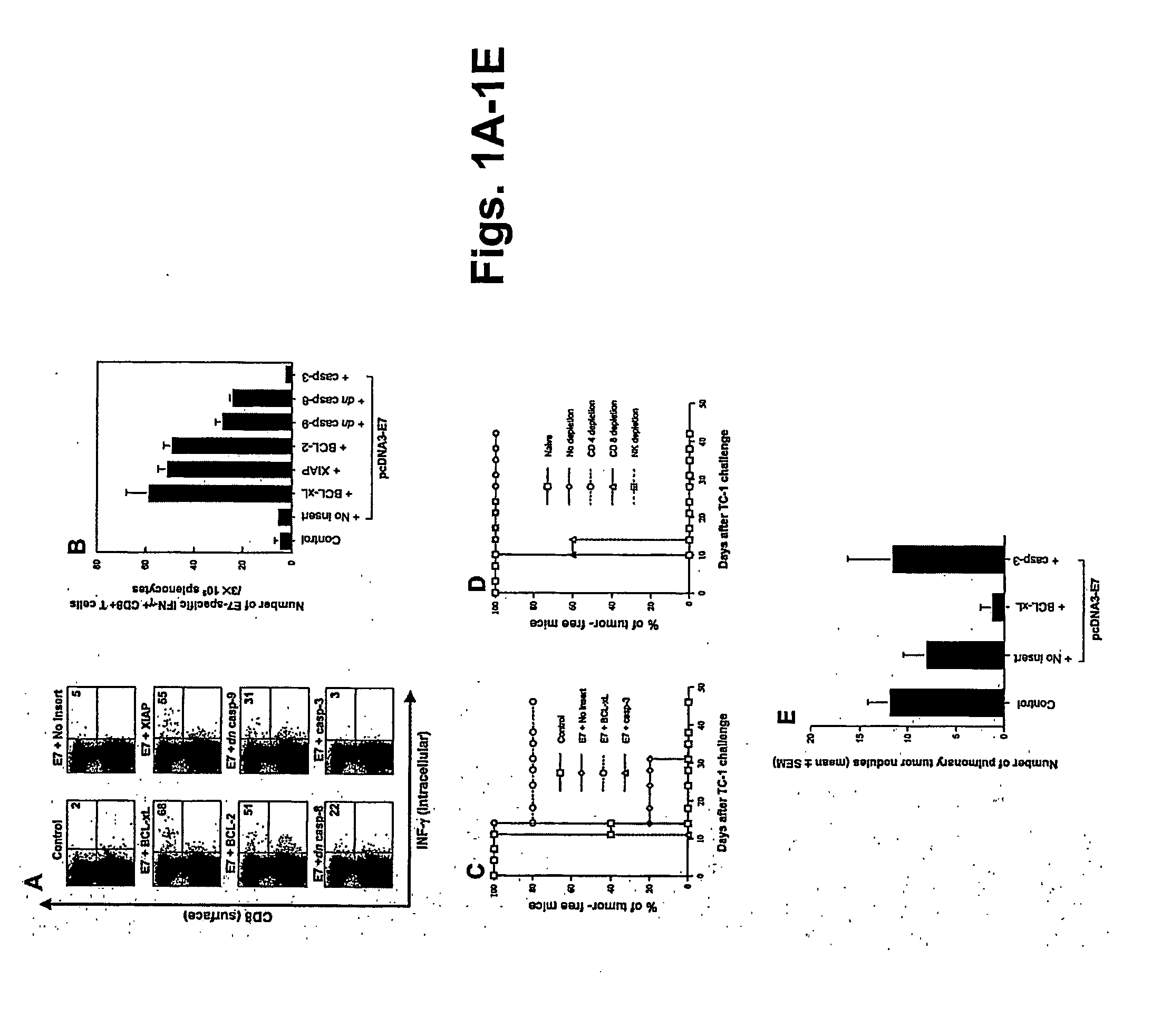
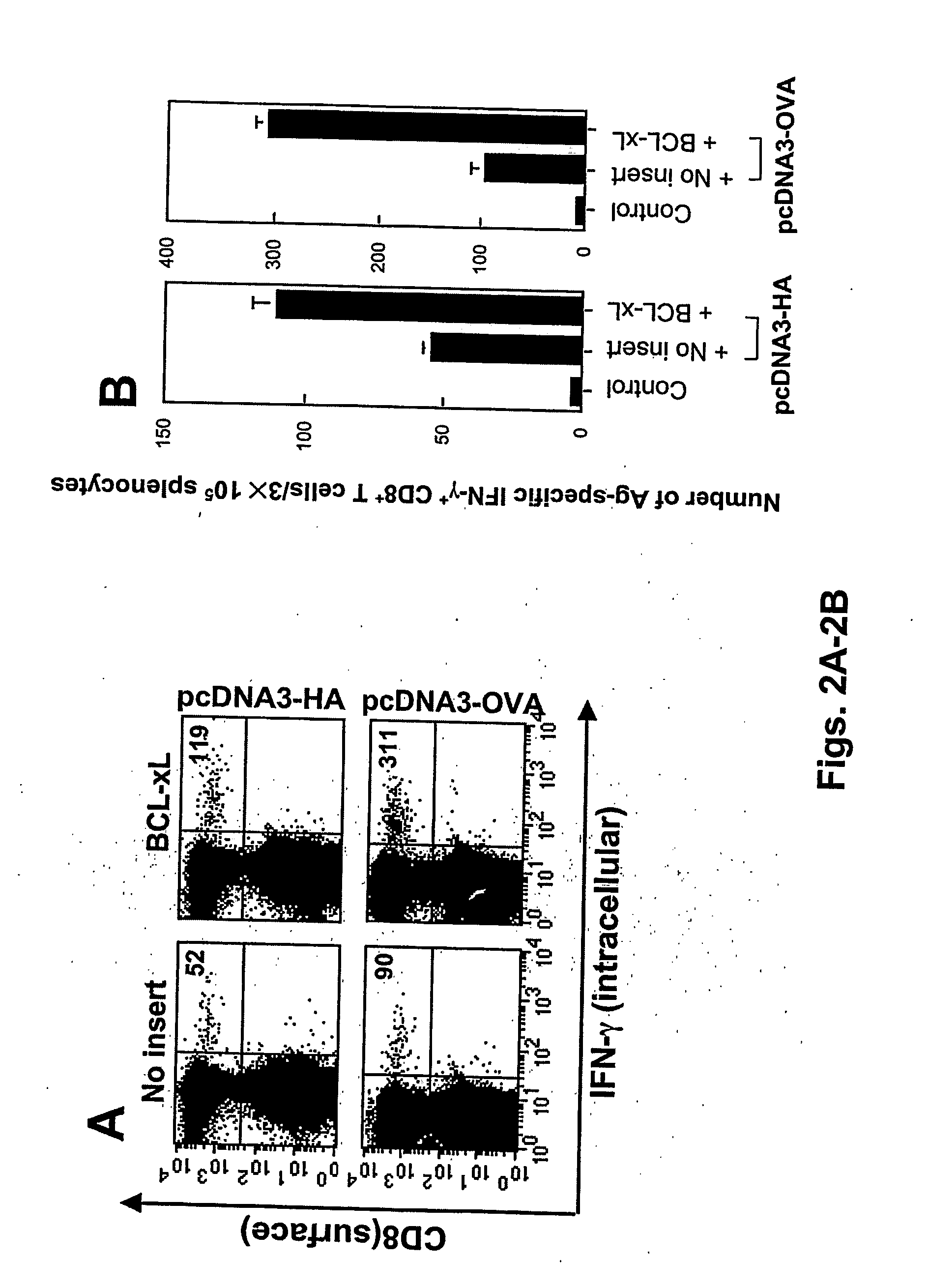
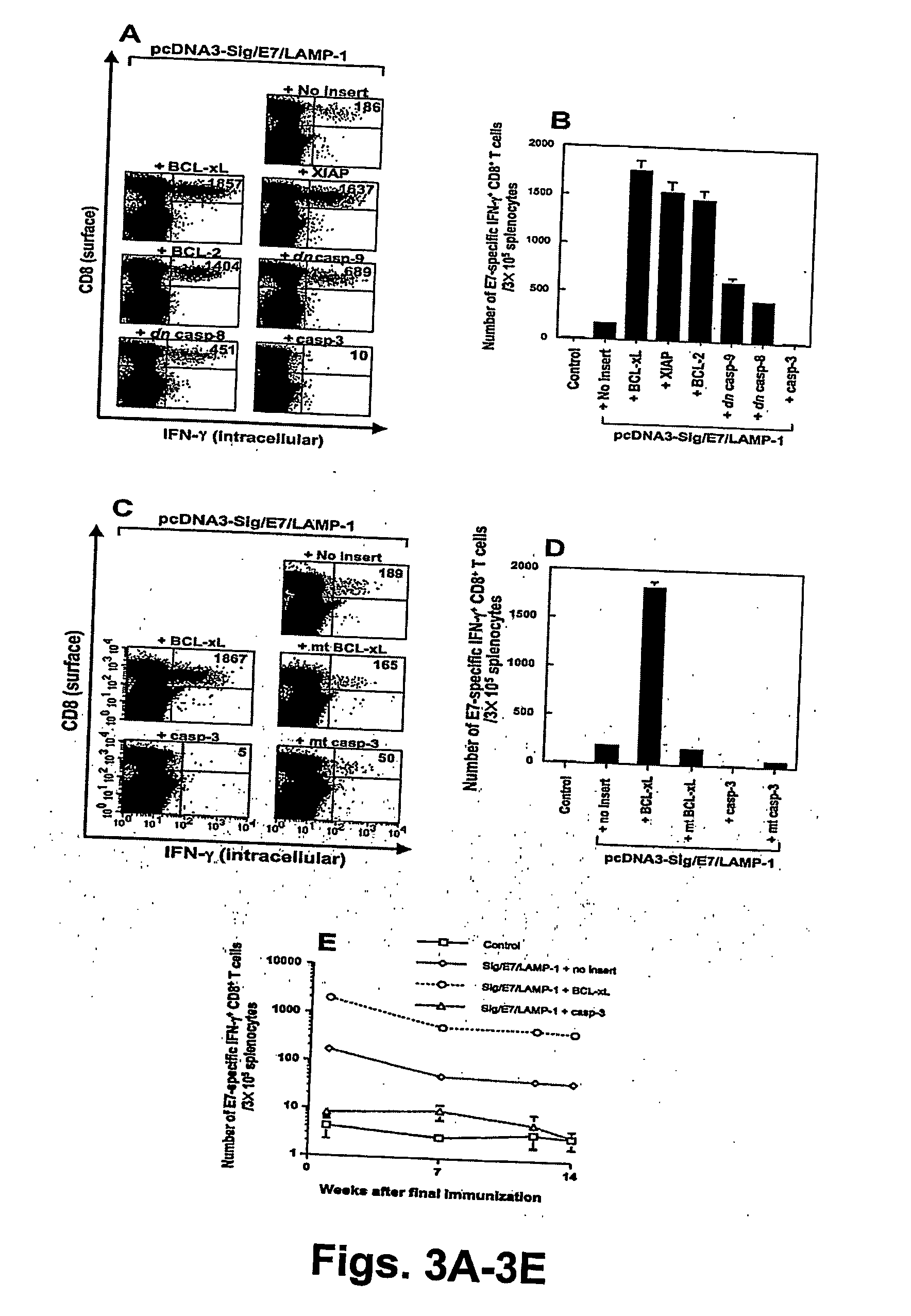
T cell immune responses are enhanced by presentation of antigen to CD8+ T cells using a chimeric nucleic acid immunogen or vaccine that links DNA encoding an antigen with DNA encoding a polypeptide that targets or translocates the antigenic polypeptide to which it is fused (immunogenicity-potentiating polypeptides or “IPP”). By inhibiting apoptosis in the vicinity of a T cell responses to such a nucleic acid immunogen, even more potent immune responses are attained. The present strategy prolongs the survival of DNA-transduced cells, including dendritic cells (DCs), thereby enhancing the priming of antigen-specific T cells and increase potency. Co-delivery of DNA encoding an inhibitor of apoptosis, including (a) BCL-xL, (b) BCL-2, (c) XIAP, (d) dominant negative caspase-9, or (e) dominant negative caspase-8, or (f) serine protease inhibitor 6 (SPI-6) which inhibits granzyme B, with DNA encoding an antigen, prolongs the survival of transduced DCs and results in significant enhancement of antigenspecific T cell immune responses that provide potent antitumor effects. Thus, co-administration of a DNA vaccine encoding antigen linked to an IPP along with one or more DNA constructs encoding an anti-apoptotic protein provides a novel way to enhance vaccine potency.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
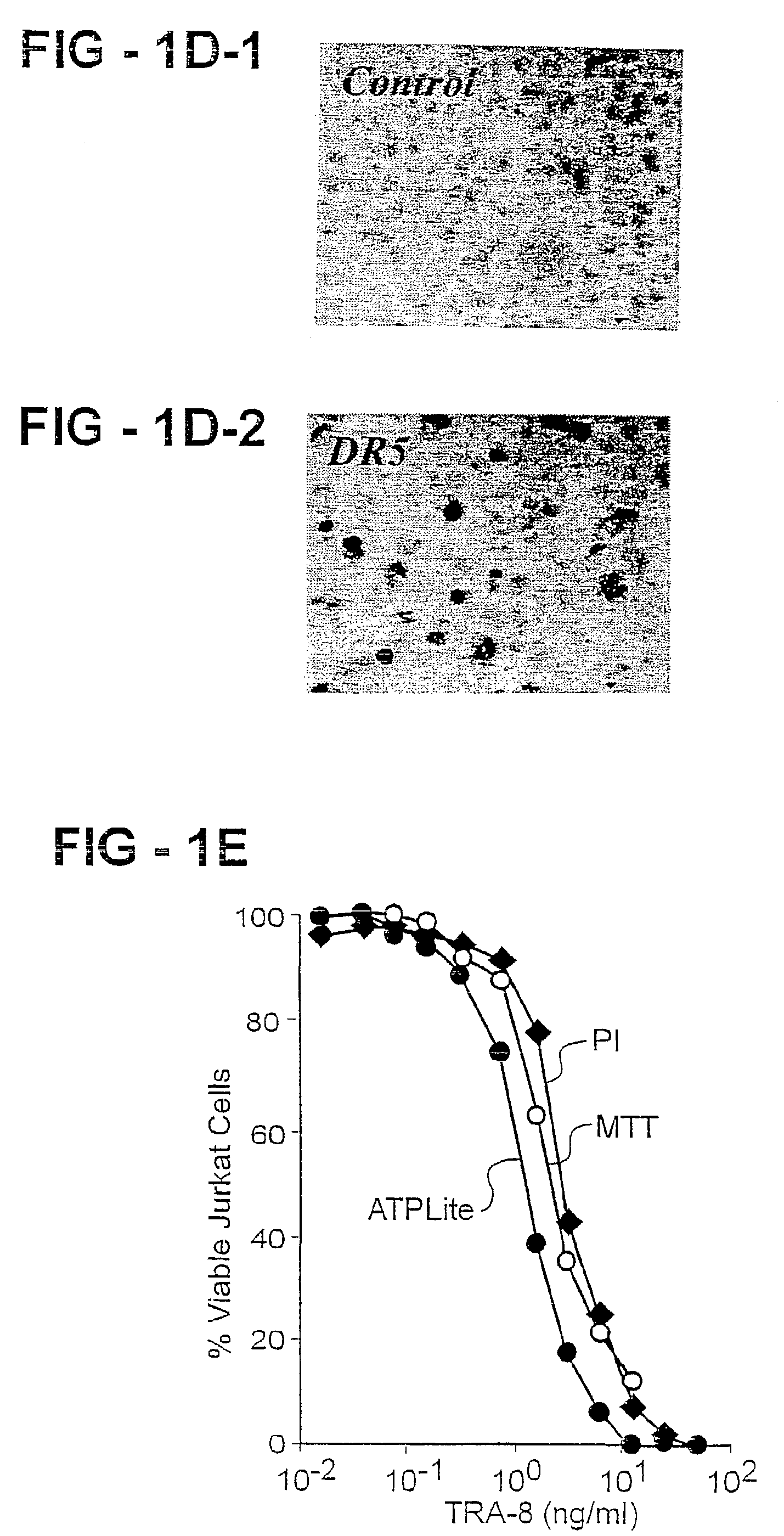
Antibody selective for a tumor necrosis factor-related apoptosis-inducing ligand receptor and uses thereof
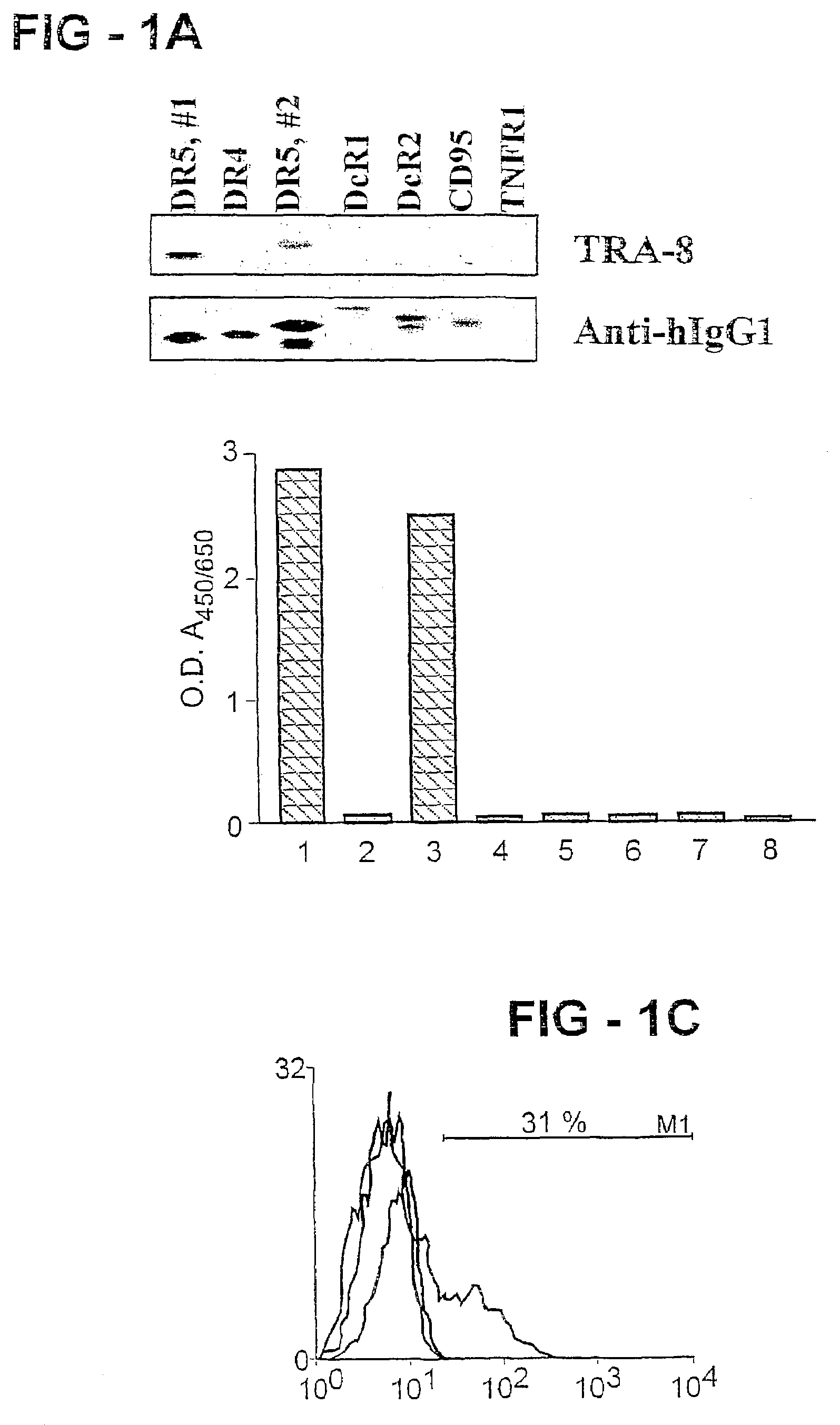
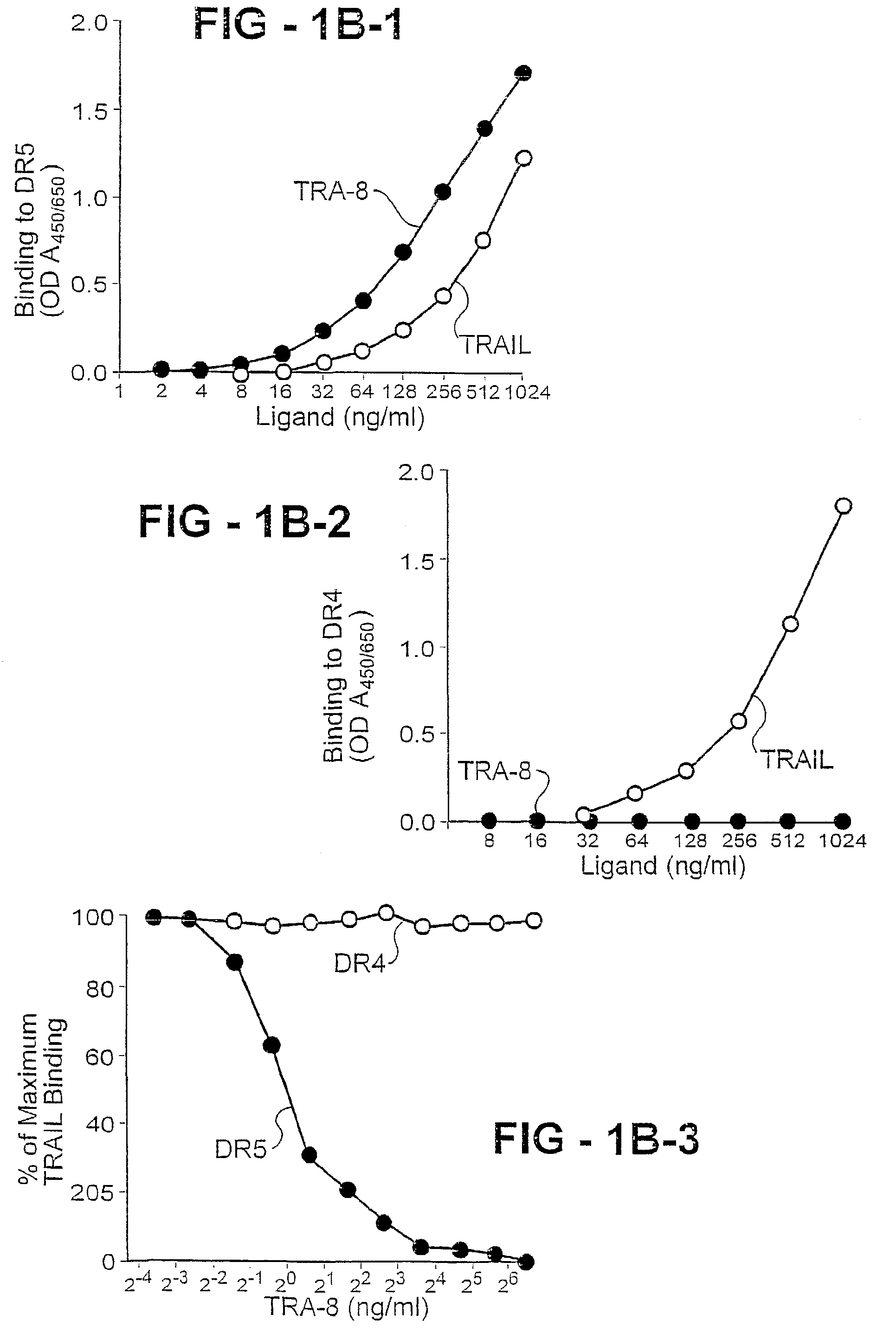
An antibody of the invention interacts with human DR5 to produce agonistic or antagonistic effects downstream of the receptor including inhibition of cell proliferation and apoptosis. Nucleic acid sequences and amino acid sequences of anti-DR5 antibodies have been elucidated and vectors and cells containing and expressing these sequences have been generated. Methods and uses for the antibodies are detailed including treatment of apoptosis-related disease and treatment of dysregulated cell growth.
Owner:UAB RES FOUND
Complex for facilitating delivery of dsRNA into a cell and uses thereof
InactiveUS20050260756A1Avoid security issuesAvoids of availabilityMicroencapsulation basedGenetic material ingredientsGene productRNA - Ribonucleic acid
The present invention provides a membrane-permeable complex for facilitating the delivery of a double-stranded ribonucleic acid molecule into a cell. Specifically, the invention provides a membrane-permeable complex that comprises a double-stranded ribonucleic acid molecule, such as a small interfering RNA, a cell-penetrating peptide, and a covalent bond linking the double-stranded ribonucleic acid to the cell-penetrating peptide. Also provided are methods of using the membrane-permeable complex of the present invention to deliver the double-stranded ribonucleic acid molecule to a cell or to inhibit expression of a gene product by a cell.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
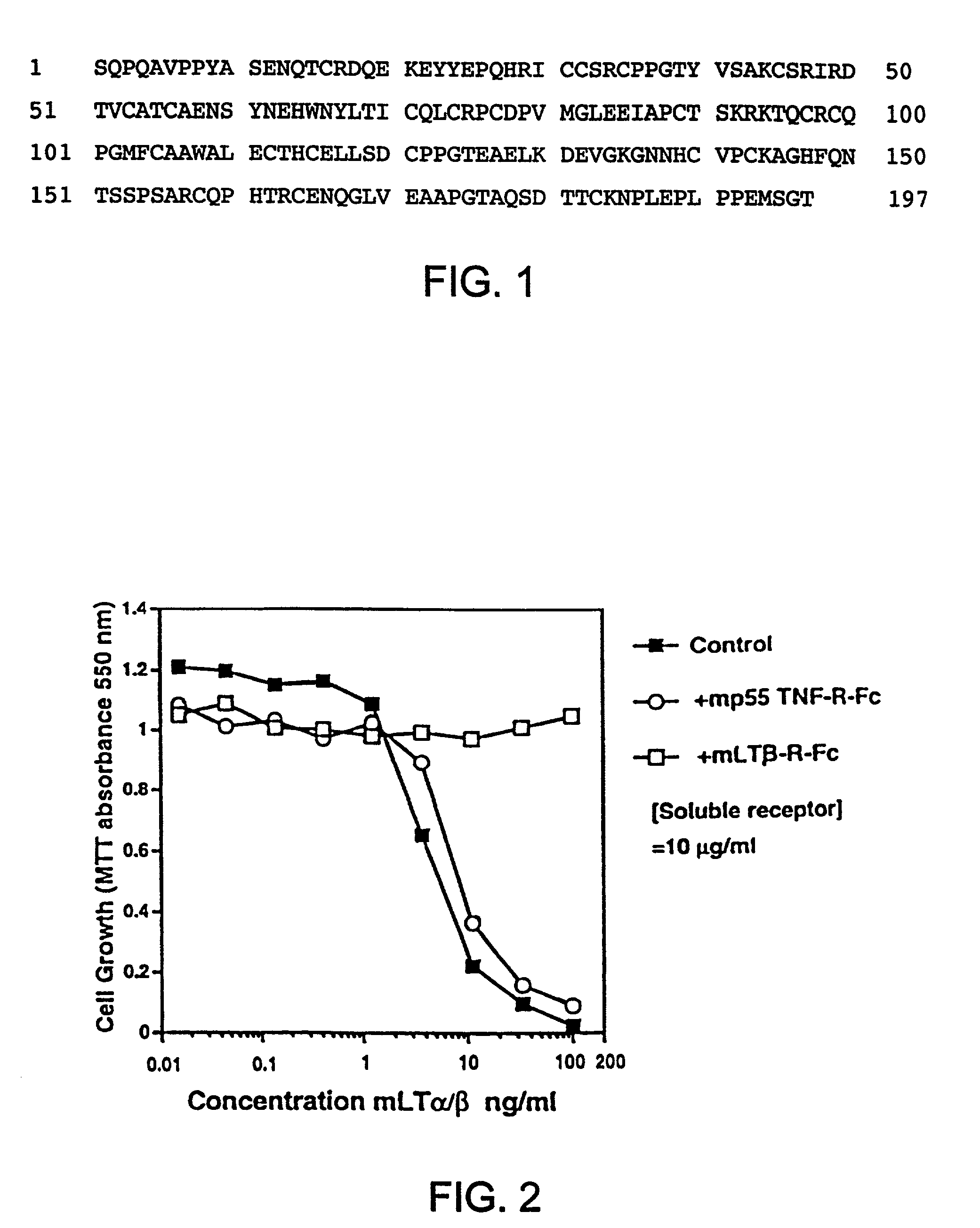
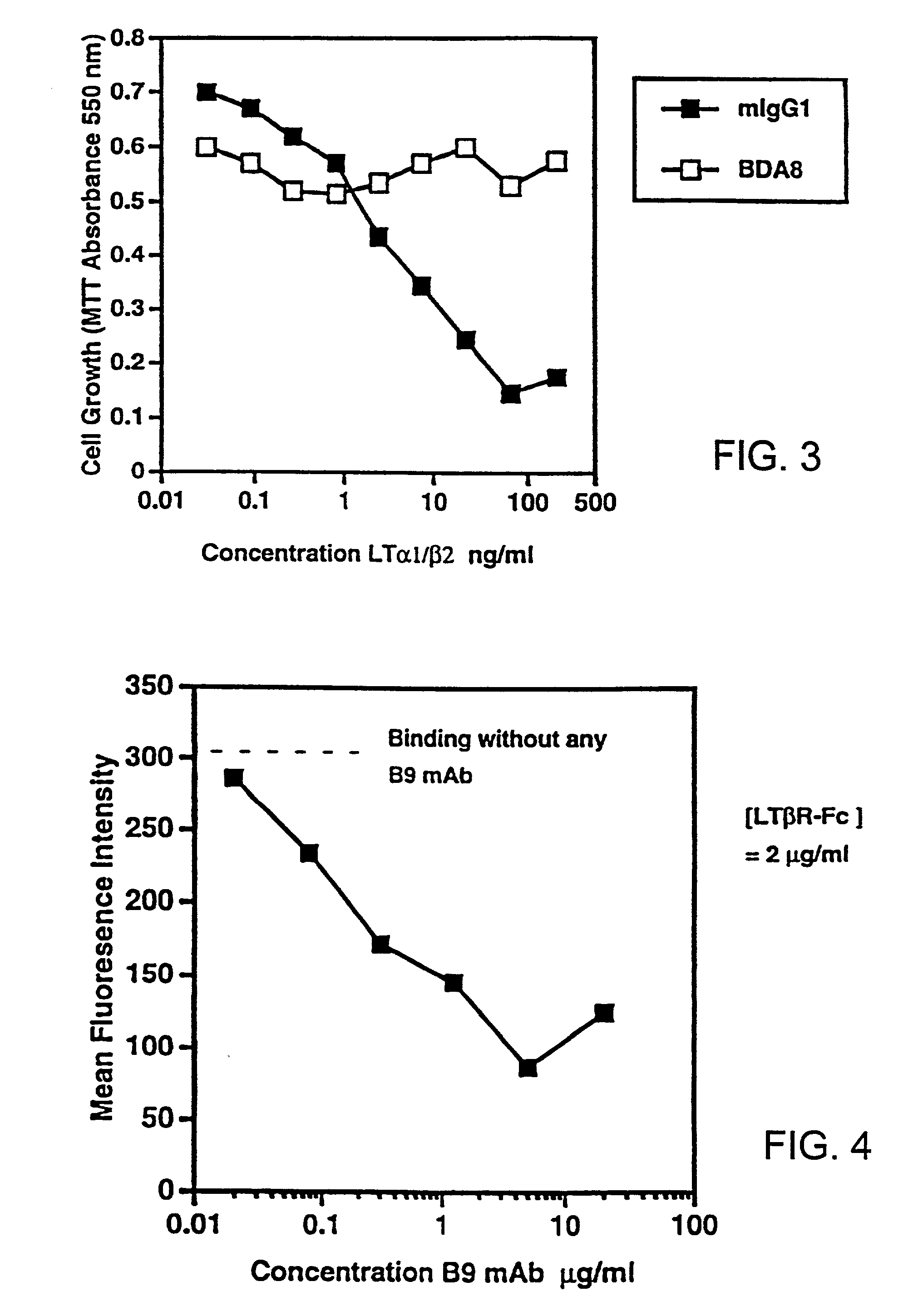
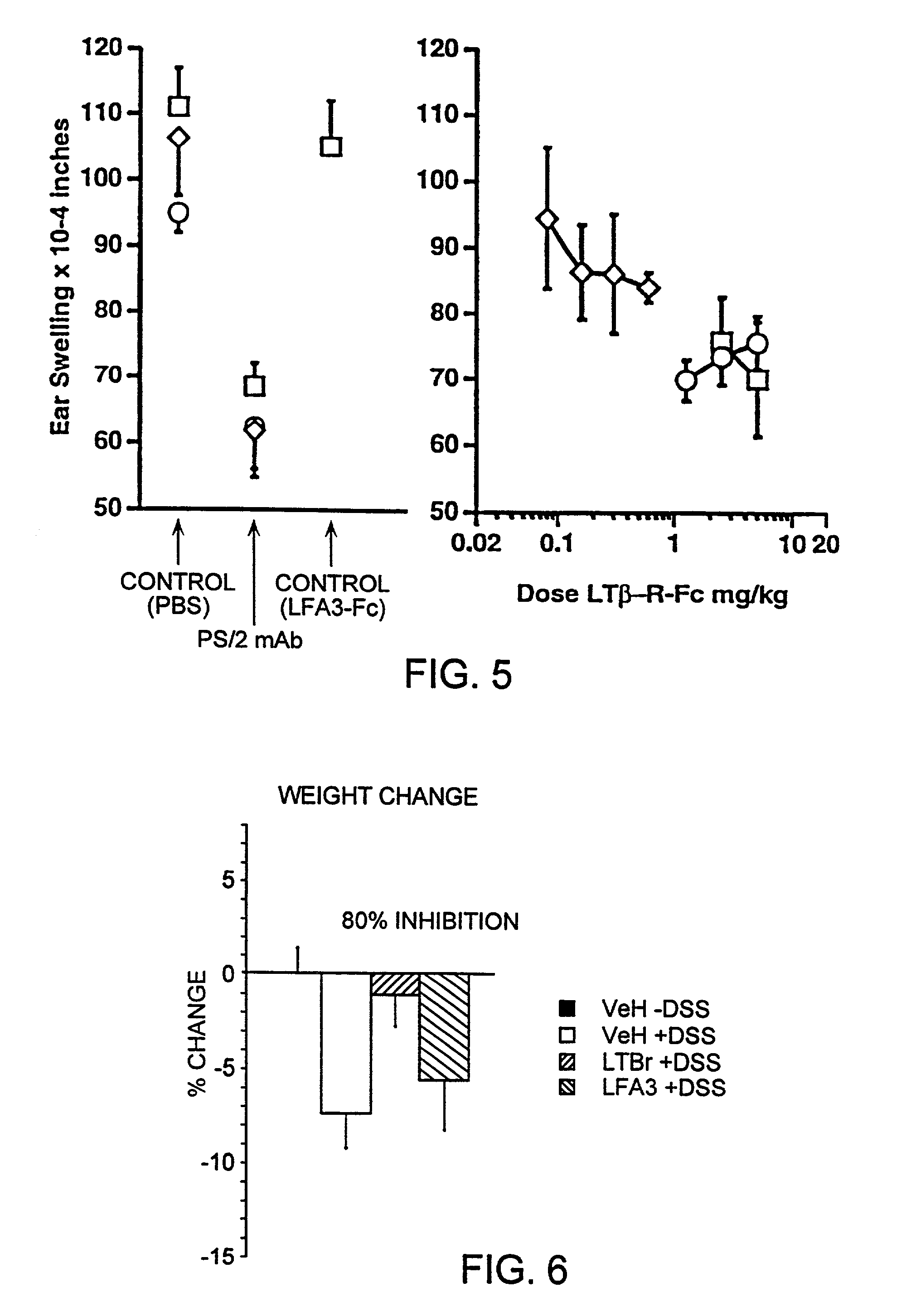
Methods for inhibiting lymphotoxin beta receptor signalling
This invention relates to compositions and methods comprising “lymphotoxin-β receptor blocking agents”, which block lymphotoxin-β receptor signalling. Lymphotoxin-β receptor blocking agents are useful for treating lymphocyte-mediated immunological diseases, and more particularly, for inhibiting Th1 cell-mediated immune responses. This invention relates to soluble forms of the lymphotoxin-β receptor extracellular domain that act as lymphotoxin-β receptor blocking agents. This invention also relates to the use of antibodies directed against either the lymphotoxin-β receptor or its ligand, surface lymphotoxin, that act as lymphotoxin-β receptor blocking agents. A novel screening method for selecting soluble receptors, antibodies and other agents that block LT-β receptor signalling is provided.
Owner:BIOGEN MA INC
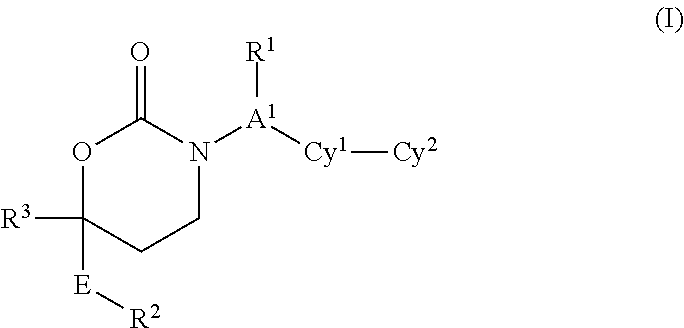
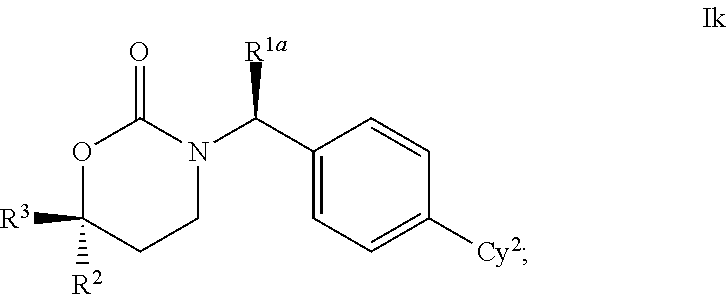
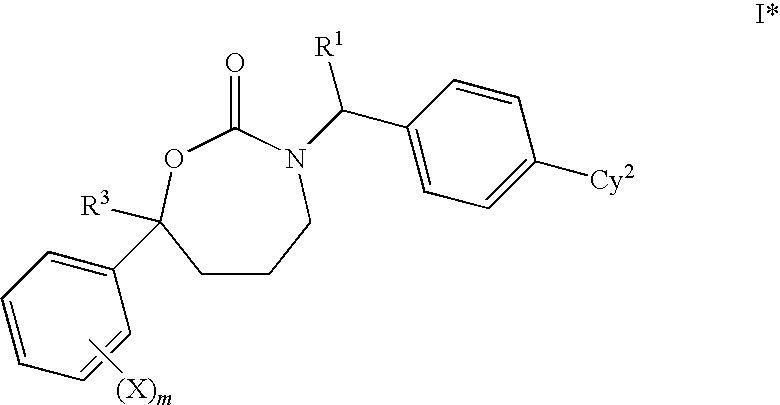
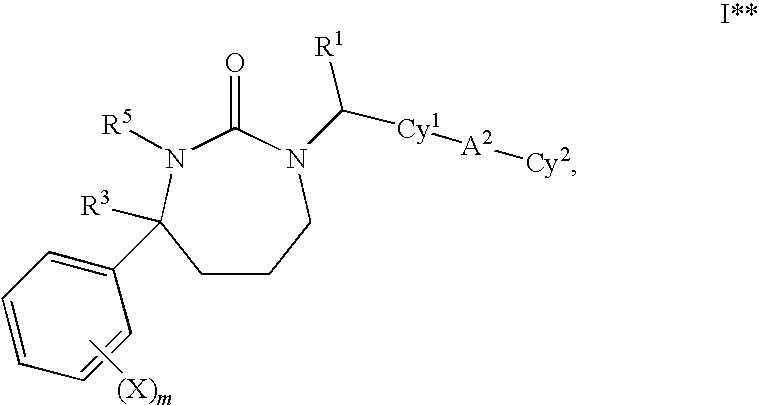
1,3-Oxazepan-2-one and 1,3-diazepan-2-one inhibitors of 11ß-hydroxysteroid dehydrogenase 1
This invention relates to novel compounds of the Formula (I), (I*), (I**), I, Ia, Ib, Ic, Id, Ie, If, Ig, Il1-3, Im1-3, In1-3, or Io1-2, pharmaceutically acceptable salts thereof, and pharmaceutical compositions thereof, which are useful for the therapeutic treatment of diseases associated with the modulation or inhibition of 11β-HSD1 in mammals. The invention further relates to pharmaceutical compositions of the novel compounds and methods for their use in the reduction or control of the production of cortisol in a cell or the inhibition of the conversion of cortisone to cortisol in a cell.
Owner:VITAE PHARMA INC
Surrogate tolerogenesis for the development of tolerance to xenografts
InactiveUS6060049AIncrease differentiationIncreased proliferationBiocideGenetic material ingredientsHematopoietic cellTolerability
PCT No. PCT / US94 / 05844 Sec. 371 Date Jun. 6, 1995 Sec. 102(e) Date Jun. 6, 1995 PCT Filed May 24, 1994 PCT Pub. No. WO94 / 27622 PCT Pub. Date Dec. 8, 1993This invention provides a method for developing immune tolerance in xenogeneic organ graft recipients, in which lympho-hematopoietic cells from an intended organ graft recipient are differentiated within a xenogeneic surrogate, such as a fetal animal. After birth of the surrogate, the matured lympho-hematopoietic cells containing antigen specific regulatory cells, including suppressor cells, veto cells, select B cells, anti-idiotype antibodies, and other related factors responsible for antigen specific tolerance in a surrogate animal are reintroduced into the intended organ graft recipient, in conjunction with an organ transplant or a tissue transplant from the xenograft surrogate. The invention also provides an organ graft repopulated with cells from the intended organ graft recipient produced in a surrogate animal.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Methods for the Treatment of Disease Using Immunoglobulins Having Fc Regions with Altered Affinities for FcgammaRactivating and FcgammaRinhibiting
ActiveUS20140134162A1Additive and synergistic and novel propertyImproved phenotypeSugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsImmunglobulin eWild type
The present invention relates to methods of treating or preventing cancer and other diseases using molecules, particularly polypeptides, more particularly immunoglobulins (e.g., antibodies), comprising a variant Fc region, wherein said variant Fc region comprises at least one amino acid modification relative to a wild-type Fc region, which variant Fc region binds an FcγR that activates a cellular effector (“FcγRActivating,” such as FcγRIIA or FcγRIIIA) and an FcγR that inhibits a cellular effector (“FcγRInhibiting,” such as FcγRIIA) with an altered Ratio of Affinities relative to the respective binding affinities of such FcγR for the Fc region of the wild-type immunoglobulin. The methods of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection where either an enhanced efficacy of effector cell function mediated by FcγR is desired (e.g., cancer, infectious disease) or an inhibited effector cell response mediated by FcγR is desired (e.g., inflammation, autoimmunde disease).
Owner:MACROGENICS INC
Inhibition of apoptosis process and improvement of cell performance
InactiveUS20030064510A1Increase productionIncreased cell performancePeptide/protein ingredientsAntibody mimetics/scaffoldsCell therapyMolecular biology
The present invention relates to preventing or delaying programmed cell death by expressing one or more anti-apoptotic polypeptides in a cell such that programmed cell death in the cell is prevented or delayed. The present invention also relates to increasing production of a cell-related product by expressing one or more anti-apoptotic polypeptides in a cell such that production of the cell-related product by the cell is increased. Recombinant cells useful for producing cell-related product or cellular therapy are also provided.
Owner:BIOGEN INC
Cyclic Inhibitors Of 11Beta-Hydroxysteroid Dehydrogenase 1
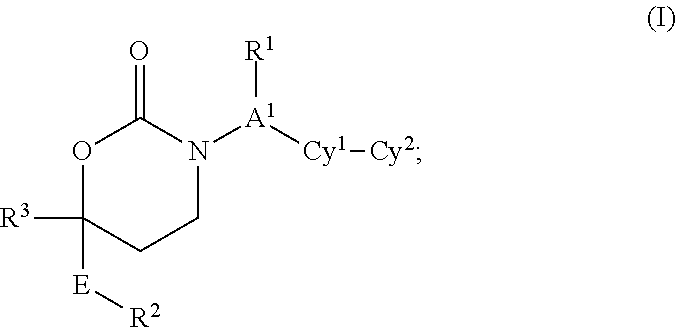
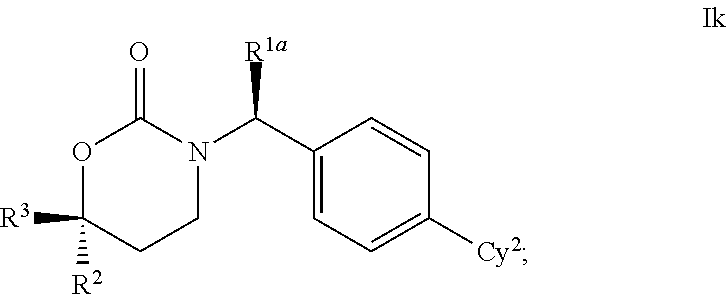
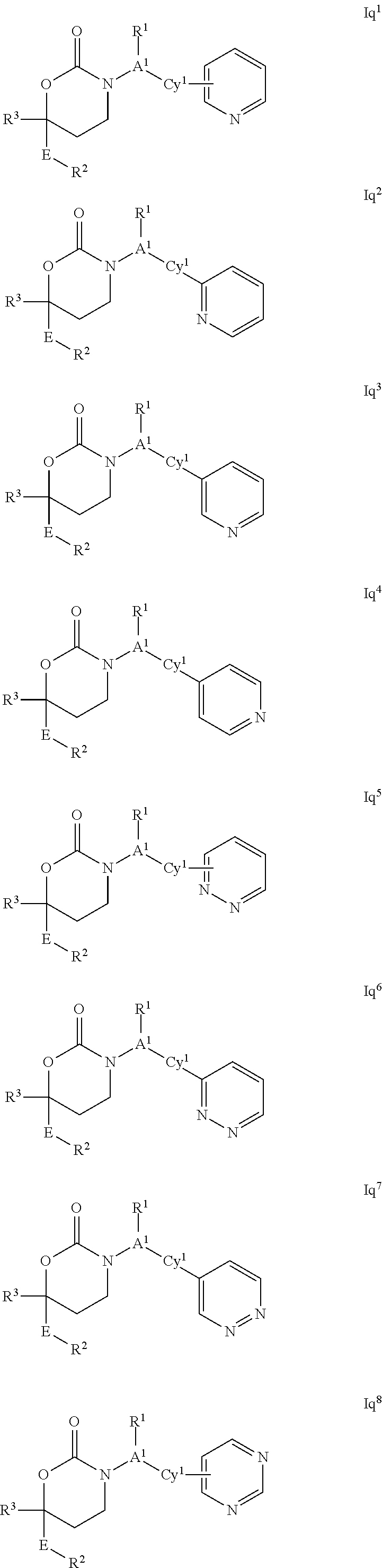
This invention relates to novel compounds of the Formula I, Ik, Iq1-21, Ir1-21, Is1-21, It1-7, pharmaceutically acceptable salts thereof, and pharmaceutical compositions thereof, which are useful for the therapeutic treatment of diseases associated with the modulation or inhibition of 11 β-HSD1 in mammals. The invention further relates to pharmaceutical compositions of the novel compounds and methods for their use in the reduction or control of the production of cortisol in a cell or the inhibition of the conversion of cortisone to cortisol in a cell.
Owner:VITAE PHARMA INC +1
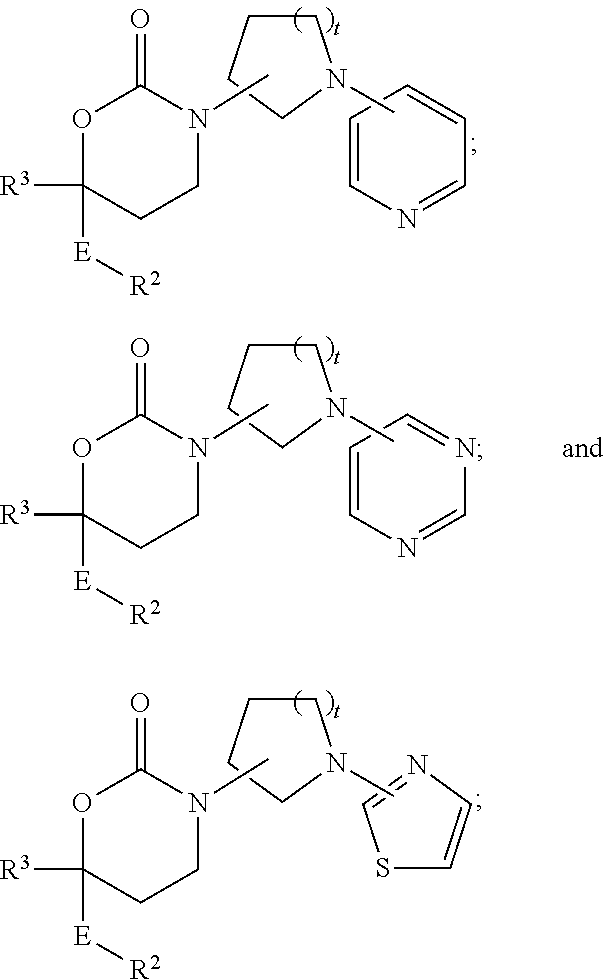
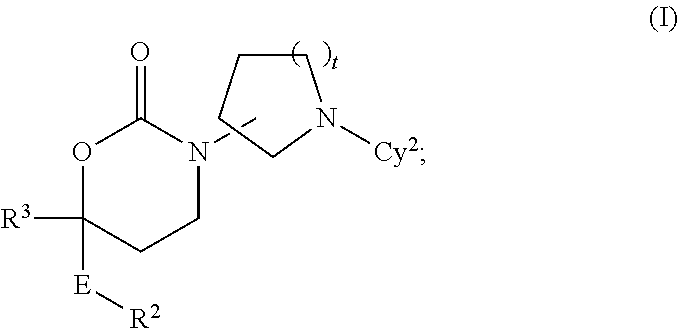
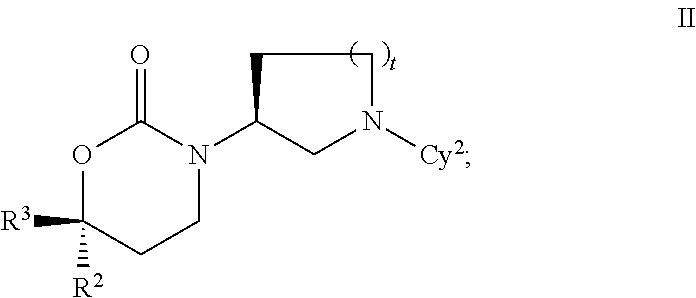
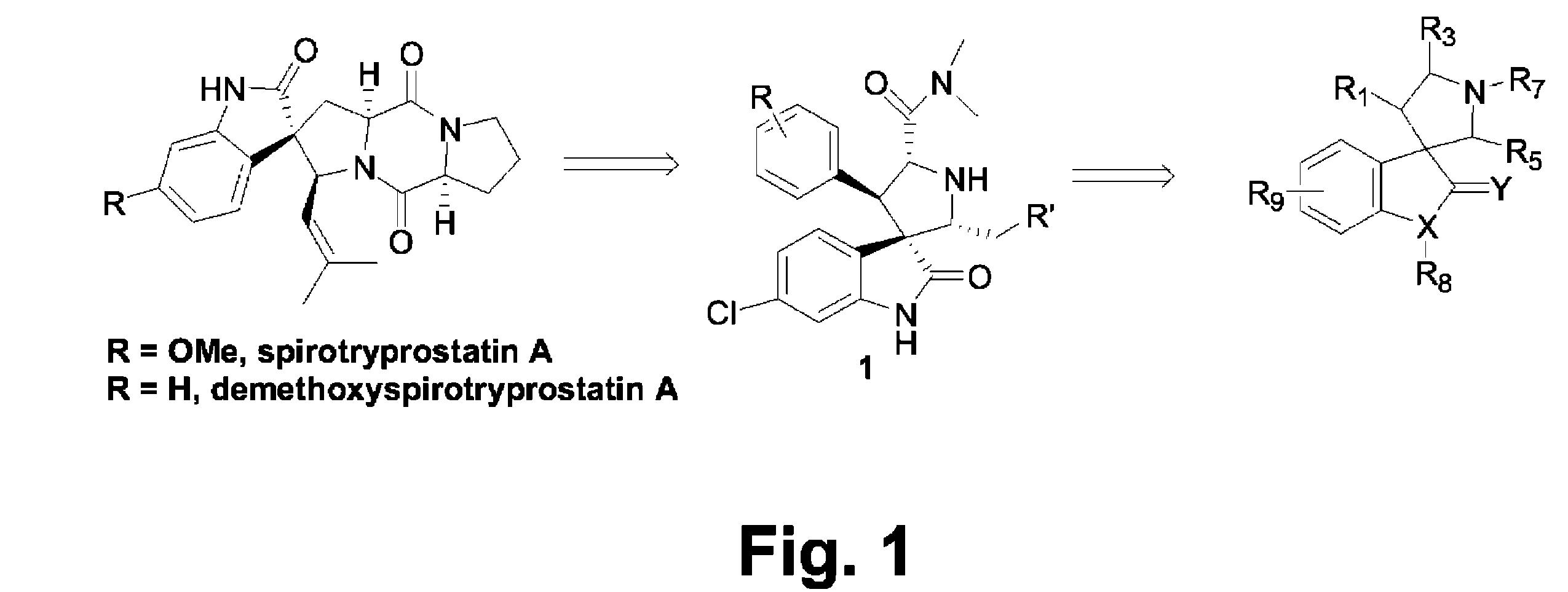
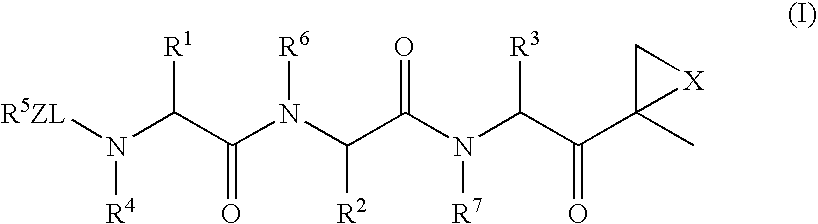
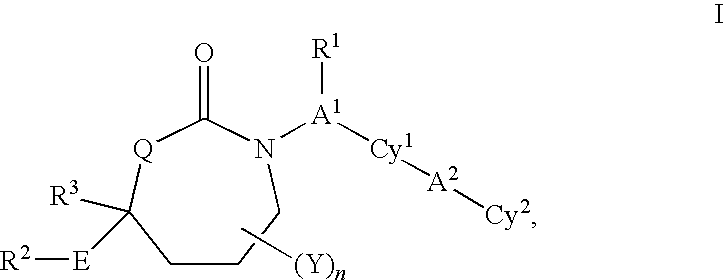
Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds
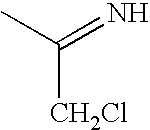
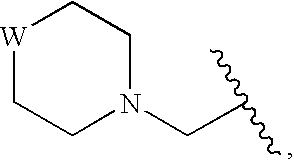
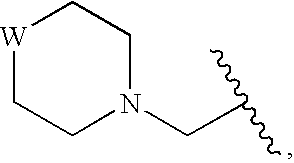
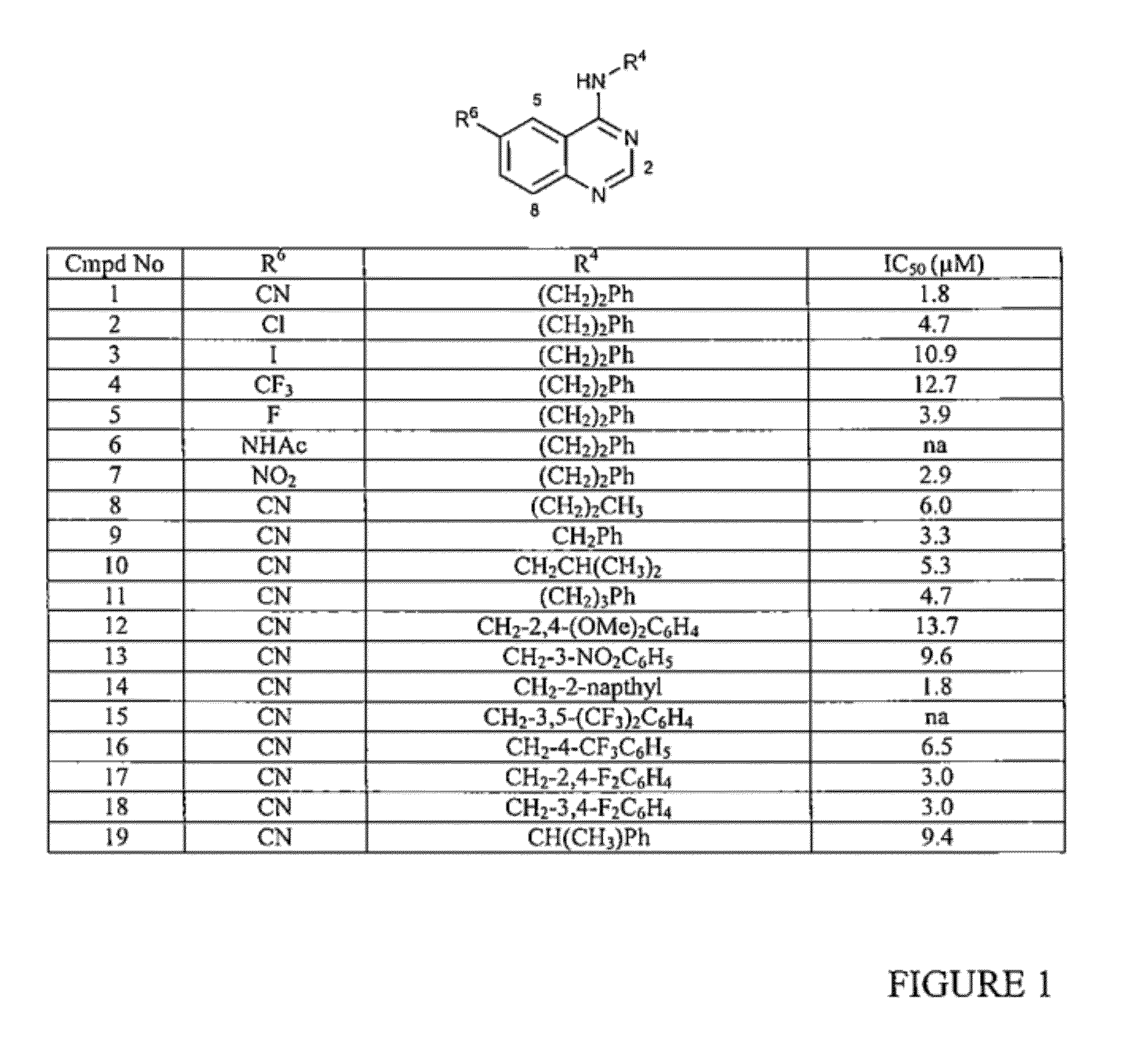
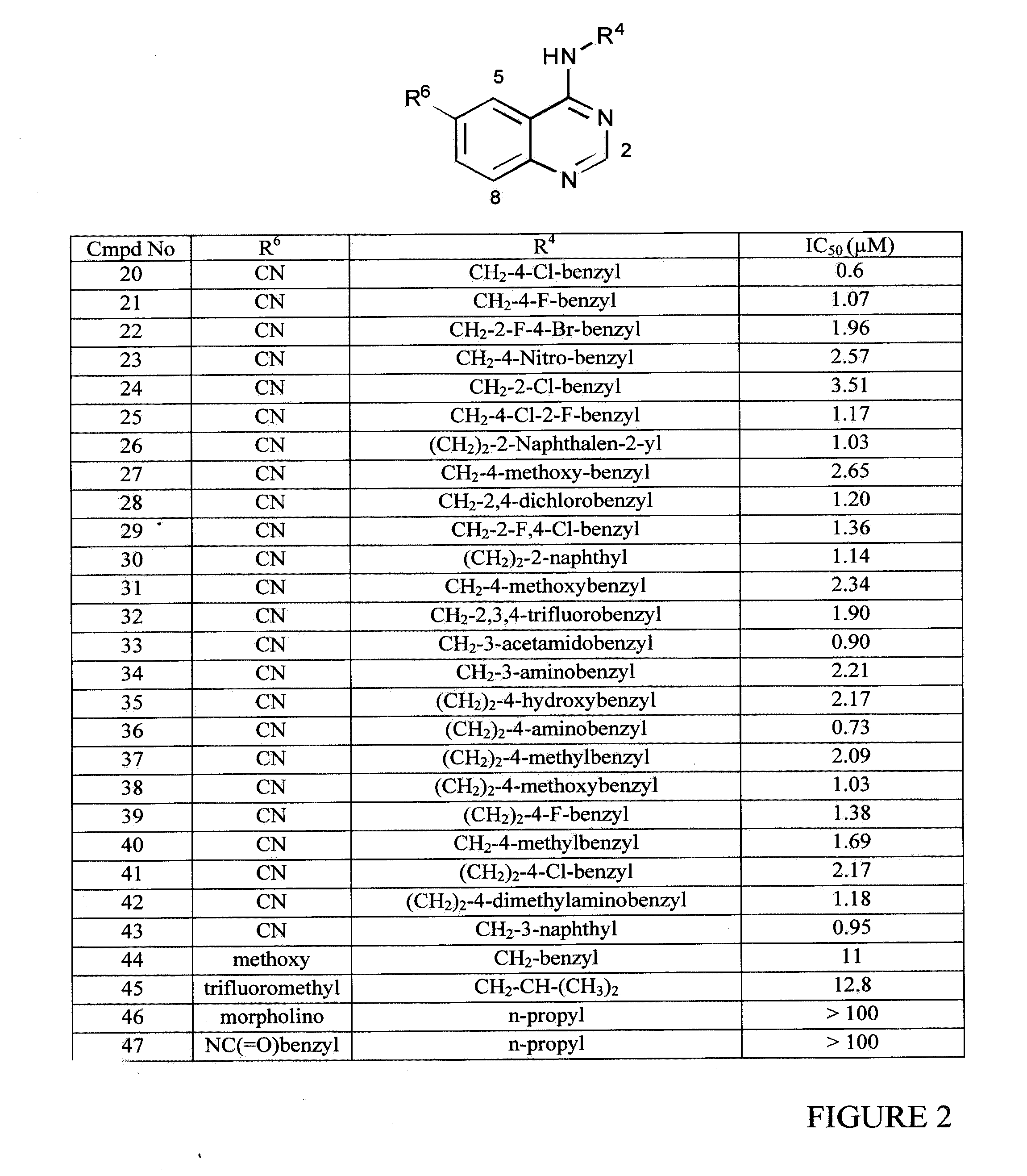
InactiveUS20090325945A1Promote apoptosisInhibiting cell cycle progressionBiocideOrganic chemistryDiseaseMelanoma
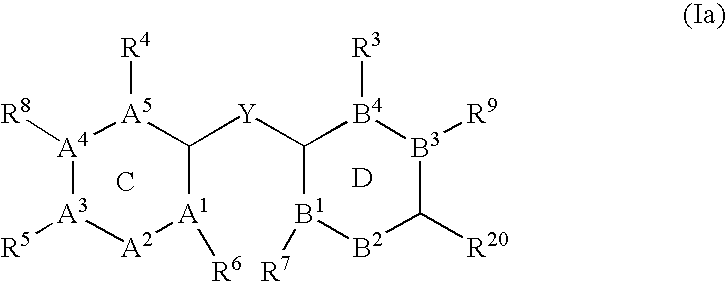
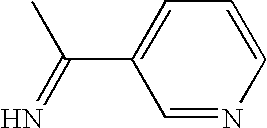
The present invention pertains to certain imidazo[4,5-b]pyridin-2-one and oxazolo[4,5 b]pyridin-2-one compounds and analogs thereof, which, inter alia, inhibit RAF (e.g., B RAF) activity, inhibit cell proliferation, treat cancer, etc., and more particularly to compounds of the formulae: wherein: J is independently —O— or —NRN1−; RN1, if present, is independently —H or a substituent; RN2 is independently —H or a substituent; Y is independently —CH═ or —N═; Q is independently —(CH2)j-M-(CH2)k— wherein: j is independently 0, 1 or 2; k is independently 0, 1, or 2; j+k is 0, 1, or 2; M is independently O—, —S—, —NH—, —NMe-, or —CH2—; each of RP1, RP2, RP5, and RP4 is independently —H or a substituent; and additionally RP1 and RP2 taken together may be CH═CH—CH═CH—; and additionally RP1 and RP5 taken together may be CH═CH—CH═CH—; L is independently: a linker group formed by a chain of 2, 3, or 4 linker moieties; each linker moiety is independently CH2—, —NRN—, —C(═X)—, or —S(═O)2—; either: exactly one linker moiety is —NRN—, or: exactly two linker moieties are —NRN—; either: exactly one linker moiety is —C(═X)—, and no linker moiety is —S(═O)2—, or: exactly one linker moiety is —S(═O)2—, and no linker moiety is —C(═X)—; no two adjacent linker moieties are —NRN—; X is independently ═O or ═S; each RN is independently —H or a substituent; A is independently: C6-14carboaryl, C5-14heteroaryl, C3-12carbocyclic, C3-12heterocyclic; and is independently unsubstituted or substituted; and pharmaceutically acceptable salts, solvates, amides, esters, ethers, N-oxides, chemically protected forms, and prodrugs thereof. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit RAF (e.g., B-RAF) activity, to inhibit receptor tyrosine kinase (RTK) activity, to inhibit cell proliferation, and in the treatment of diseases and conditions that are ameliorated by the inhibition of RAF, RTK, etc., proliferative conditions such as cancer (e.g., colorectal cancer, melanoma), etc.
Owner:CANCER RES TECH LTD +1
Combination of kinase inhibitors and uses thereof
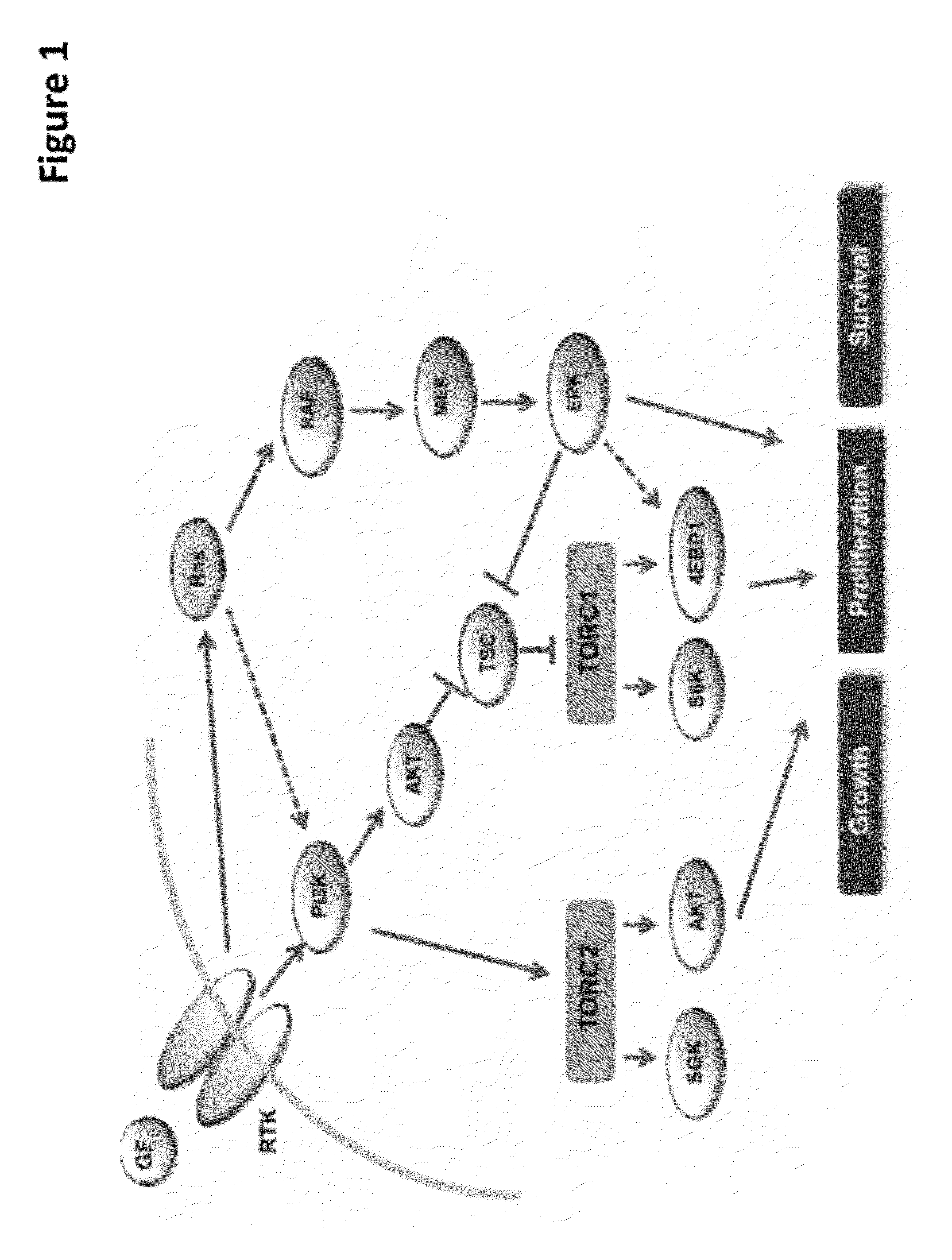
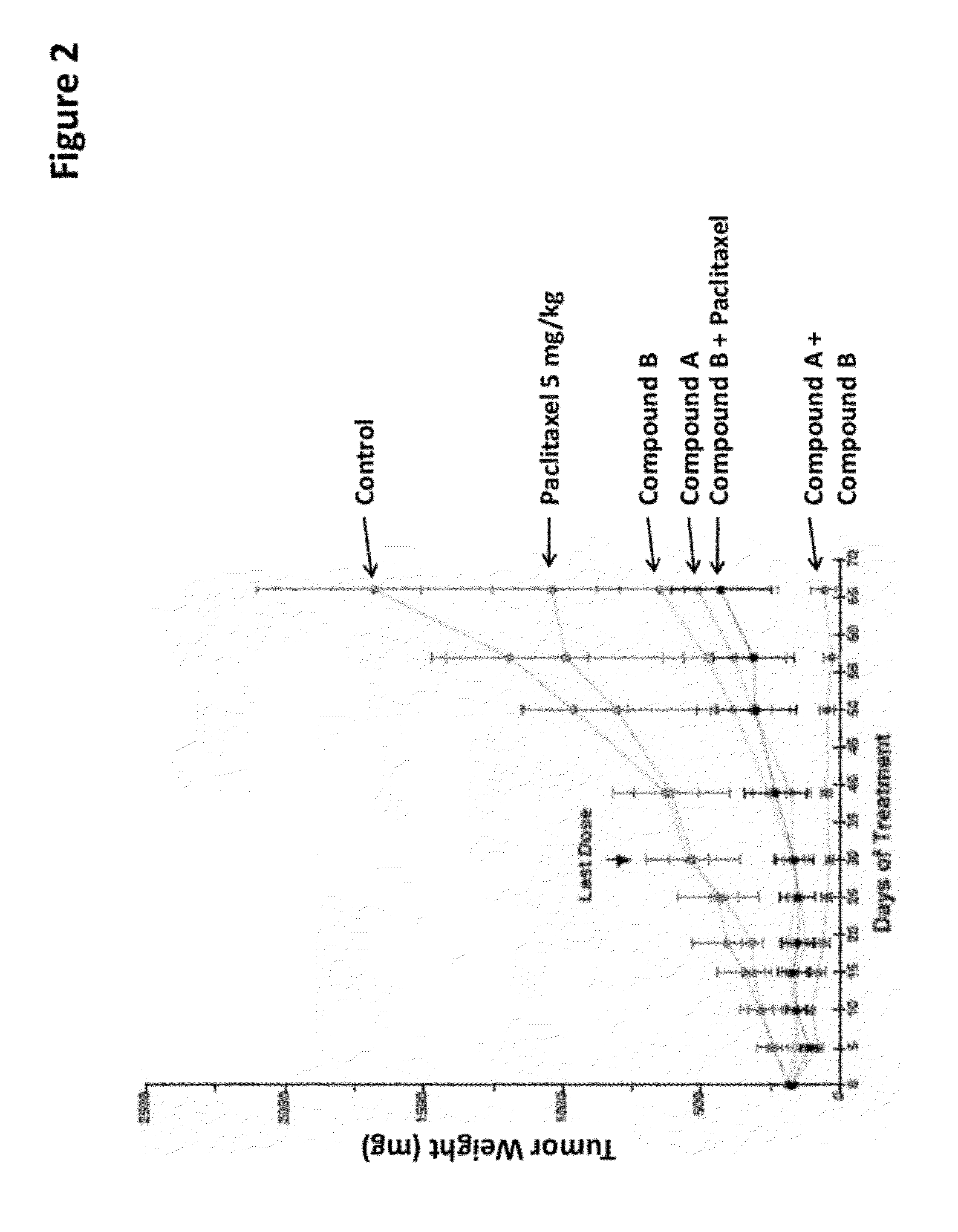
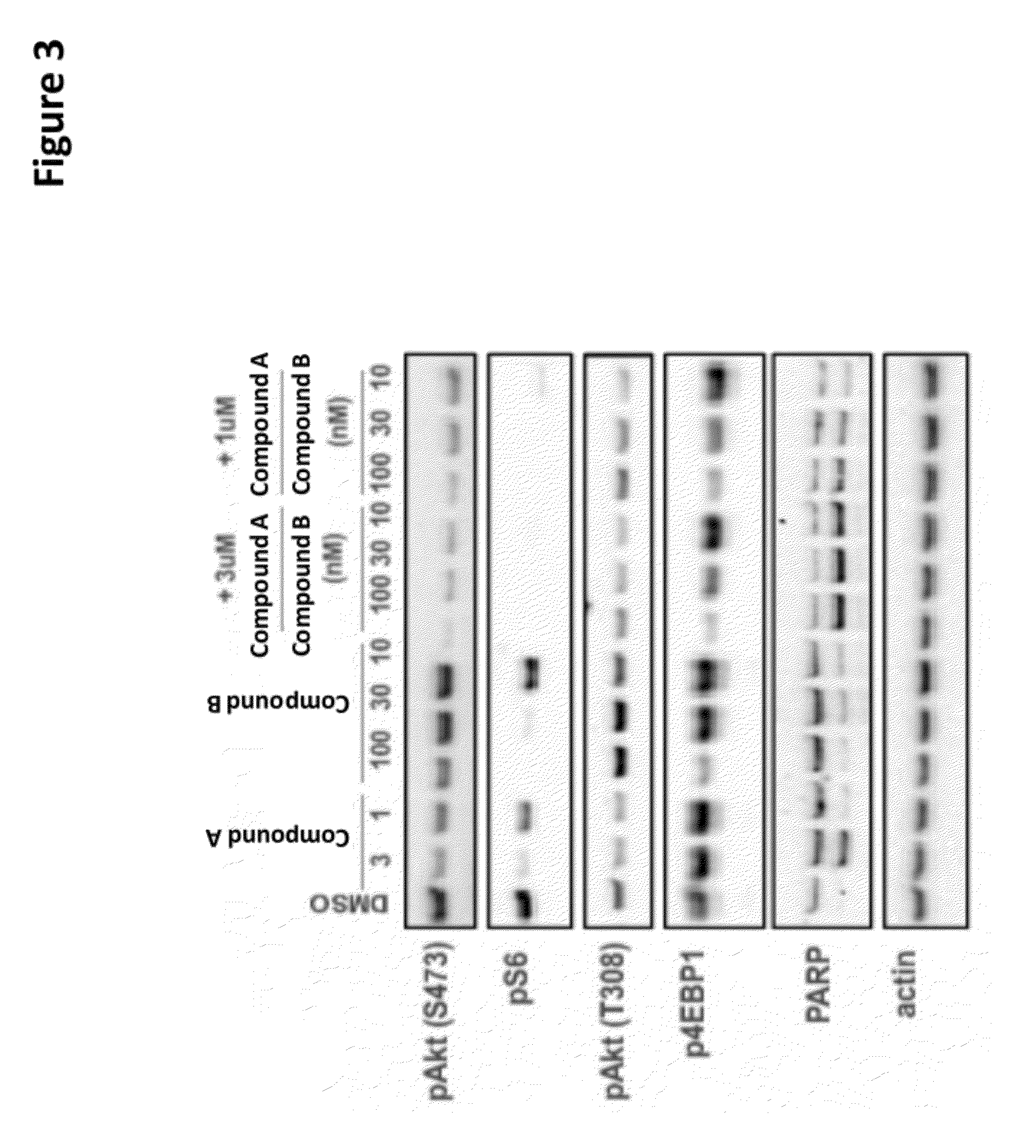
The present invention provides for a method for treating a disease condition associated with PI3-kinase α and / or mTOR in a subject. In another aspect, the invention provides for a method for treating a disease condition associated with PI3-kinase α and / or mTOR in a subject. In yet another aspect, a method of inhibiting phosphorylation of both Akt (S473) and Akt (T308) in a cell is set forth.
Owner:INTELLIKINE
Cyclic inhibitors of 11β-hydroxysteroid dehydrogenase 1
This invention relates to novel compounds of a structural formula selected from:pharmaceutically acceptable salts thereof, and pharmaceutical compositions thereof, which are useful for the therapeutic treatment of diseases associated with the modulation or inhibition of 11β-HSD1 in mammals, e.g., diabetes mellitus and obesity. Values for the variables in the structural formulas are provided herein. The invention further relates to pharmaceutical compositions of the novel compounds and methods for their use in the reduction or control of the production of cortisol in a cell or the inhibition of the conversion of cortisone to cortisol in a cell.
Owner:VITAE PHARMA INC
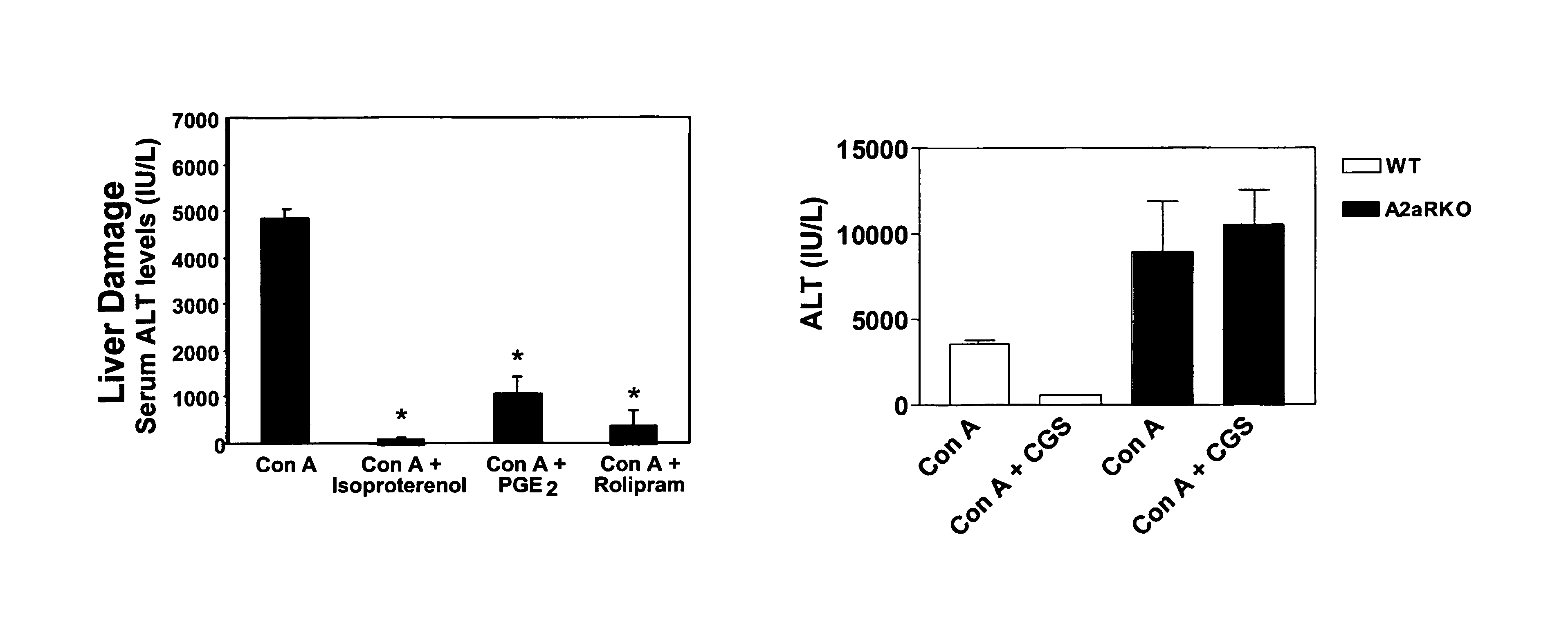
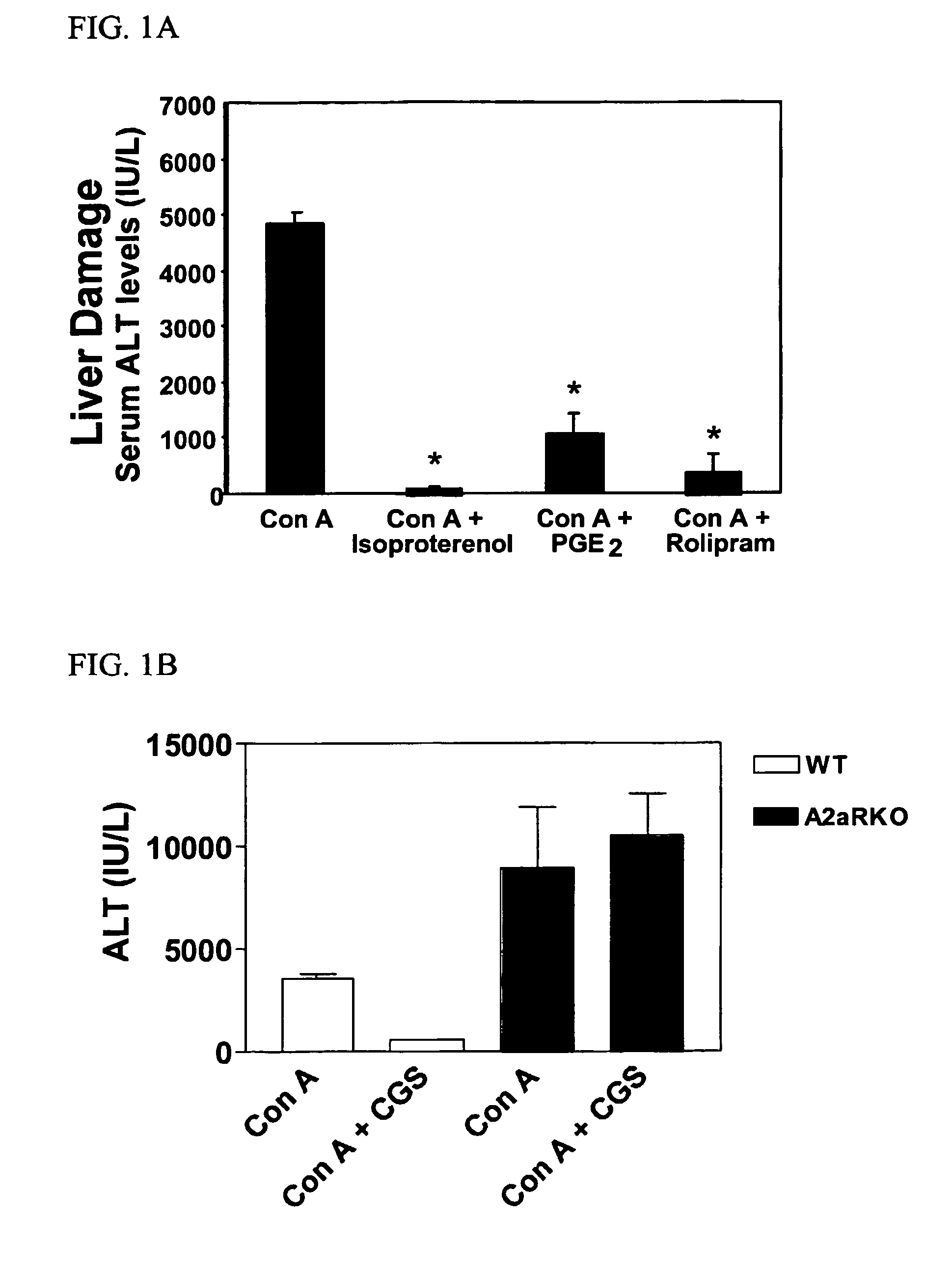
Methods for using extracellular adenosine inhibitors and adenosine receptor inhibitors to enhance immune response and inflammation
ActiveUS8080554B2Suppress immune responseEnhance immune responseAntibacterial agentsBiocideVaccine PotencyTumor destruction
A method is provided herein to increase an immune response to an antigen. The method includes administering an agent that inhibits extracellular adenosine or inhibits adenosine receptors. Also disclosed are methods to increase the efficacy of a vaccine and to increase an immune response to a tumor antigen or immune cell-mediated tumor destruction.
Owner:HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US SEC THE DEPT OF
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com





![Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds](https://images-eureka.patsnap.com/patent_img/160c93b4-7535-4186-a3b0-42b5846b0524/US20090325945A1-C00001.png)
![Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds](https://images-eureka.patsnap.com/patent_img/160c93b4-7535-4186-a3b0-42b5846b0524/US20090325945A1-C00002.png)
![Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds](https://images-eureka.patsnap.com/patent_img/160c93b4-7535-4186-a3b0-42b5846b0524/US20090325945A1-C00003.png)