Halogenated diarylurea compounds and application thereof in preparation of antiallergic drugs
A technology of halogenated diaryl urea and diaryl urea, which is applied in drug combination, allergic diseases, organic chemistry, etc., can solve the problems of unreported anti-allergic research, and achieve the enrichment of anti-allergic drug types, The effect of suppressing anaphylaxis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A class of halogenated halogenated diaryl urea compounds disclosed in the present invention is compound 10-20. The compound structural formula is shown in Table 1 above, and the structural analysis NMR data of compound 10-compound 20 are as follows:
[0045] Compound 10: Dissolve bis(trichloromethyl)carbonate (BTC) (0.25g, 0.83mmol) in 20mL of anhydrous dichloromethane under ice bath conditions and stir for 5min, slowly add 3-bromo-5- The dichloromethane solution of trifluoromethylaniline (0.5g, 2.08mmol) was stirred for 15min after the dropwise addition was completed, and the dichloromethane solution of triethylamine (0.25g, 2.50mmol) was continuously dropped into the cloudy solution. Stir for 15min, then add dropwise a dichloromethane solution of 3-bromo-5-trifluoromethylaniline (0.5g, 2.08mmol) and triethylamine (0.25g, 2.50mmol) to the reaction solution, and stir after the addition is complete 20min, the reaction solution was successively washed with saturated NaHCO...
Embodiment 2
[0057] 1. Experimental materials
[0058] Instrument: Automatic microplate reader was purchased from Bio-Rad (California, USA).
[0059] Cell line: KU812 was cultured in IMEM medium with 10% serum, plus 1:100 penicillin-streptomycin. The medium was half-replaced with fresh medium every other day to maintain the cells at 2 × 10 6 density of cells per milliliter.
[0060] Main reagents: TM buffer solution (composition (g / L): 6.954g NaCl, 0.353g KCl, 2.383g HEPES, 0.162g KH 2 PO 4 ,0.282g CaCl 2 , 0.143 MgSO 4 , 0.991g glucose, 1g bovine serum albumin. 0.1% Triton X-100 lysate; 1mmol / L β-hexosamine solution; 0.1mol / L Na 2 CO 3 / NaHCO 3 Stop solution (pH11.0).
[0061] 2. Experimental method
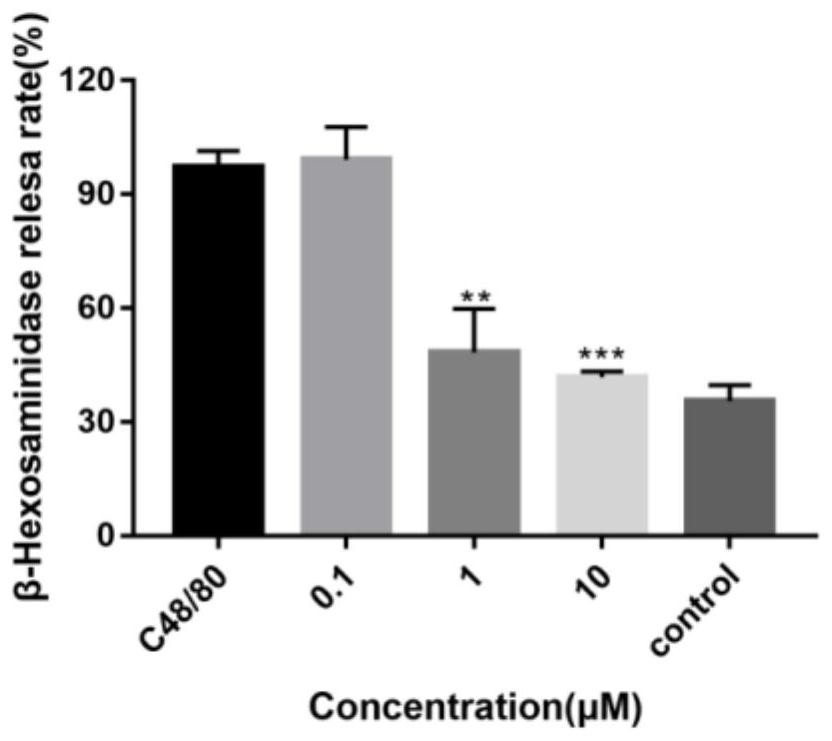
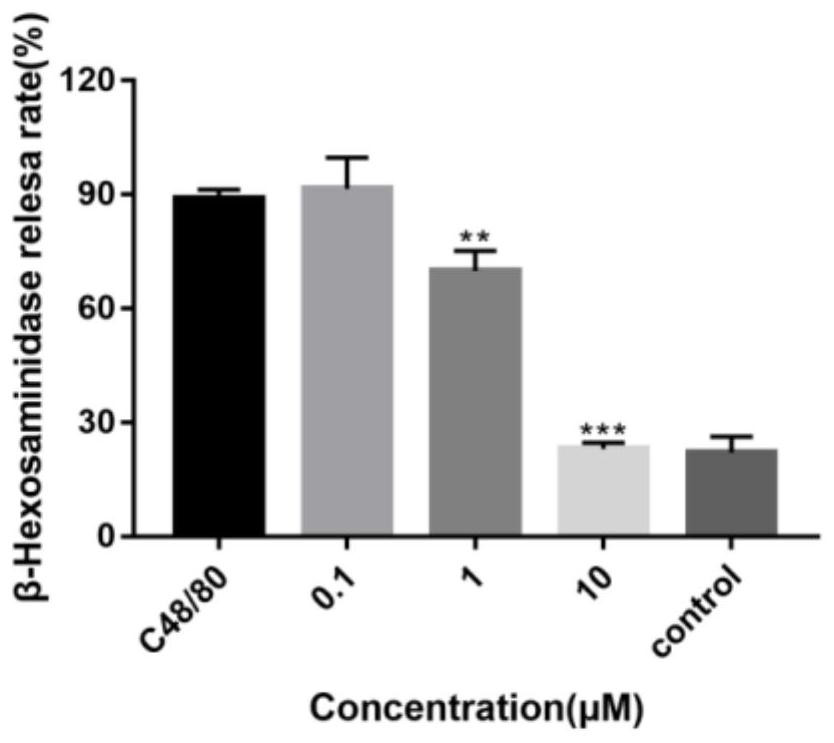
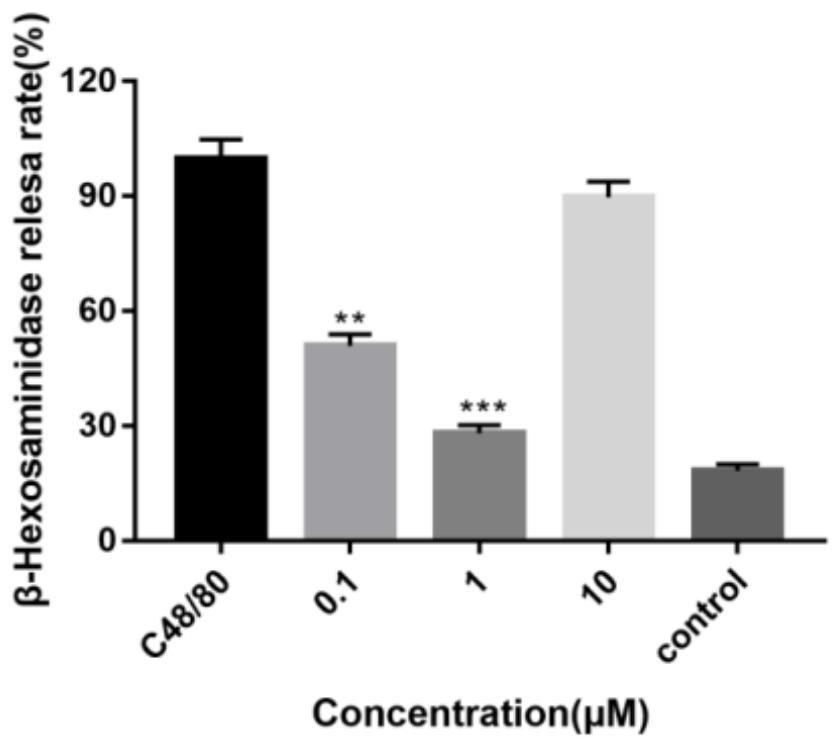
[0062] KU812 cells were seeded in 96-well plates at 30,000 cells / well and cultured overnight. After the 96-well plate was centrifuged at 1500rpm for 5 minutes, the original medium was discarded, and different drugs were added according to the preset groupings: 90 μL TM buffer was a...
Embodiment 3
[0070] 1. Experimental materials
[0071] Instrument: LCMS-8040 triple quadrupole liquid chromatography mass spectrometry (Shimadzu, Japan)
[0072] Cell line: KU812 was cultured in IMEM medium with 10% serum, plus 1:100 penicillin-streptomycin. The medium was half-replaced with fresh medium every other day to maintain the cells at 2 × 10 6 density of cells per milliliter.
[0073] Basic chromatographic conditions: chromatographic column: Venusil HILIC column (3μm, 2.1×150mm); mobile phase is acetonitrile (0.1% formic acid): 20mM ammonium formate water = 80:20; flow rate: 0.3mL / min; column temperature: 35°C.
[0074] 2. Experimental method
[0075] KU812 cells were seeded in 96-well plates at 30,000 cells / well and cultured overnight. The 96-well plate was centrifuged at 1500 rpm for 5 minutes, and the culture medium was discarded by suction. Add 50 μL of prepared drugs with a series of concentrations to the administration group; add 50 μL TM buffer to the blank group and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com