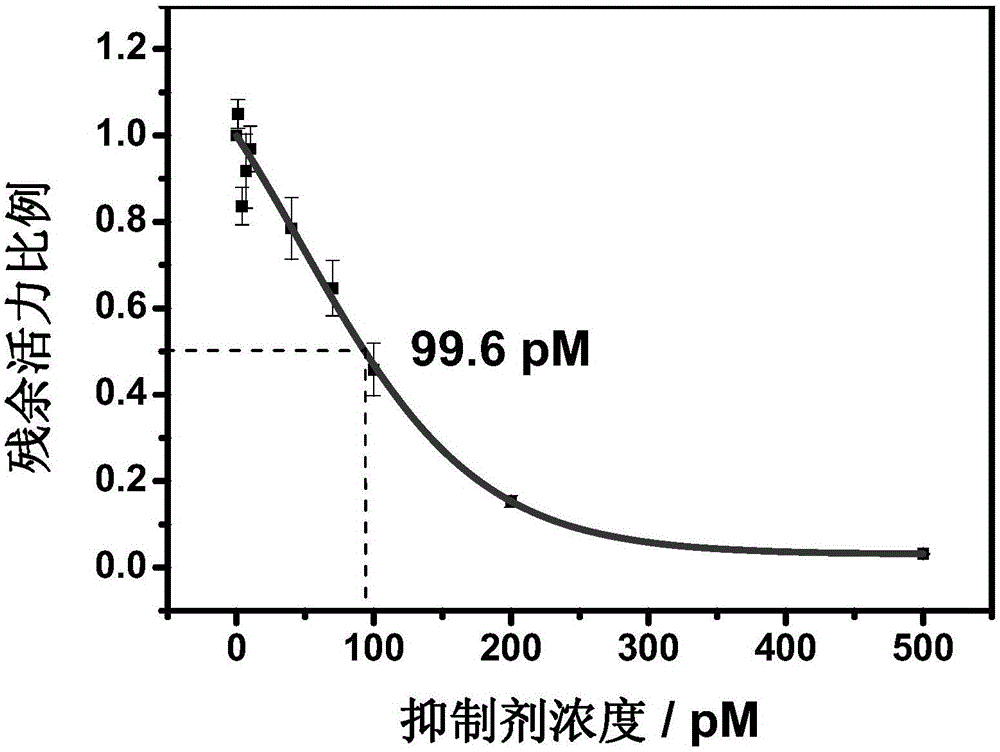
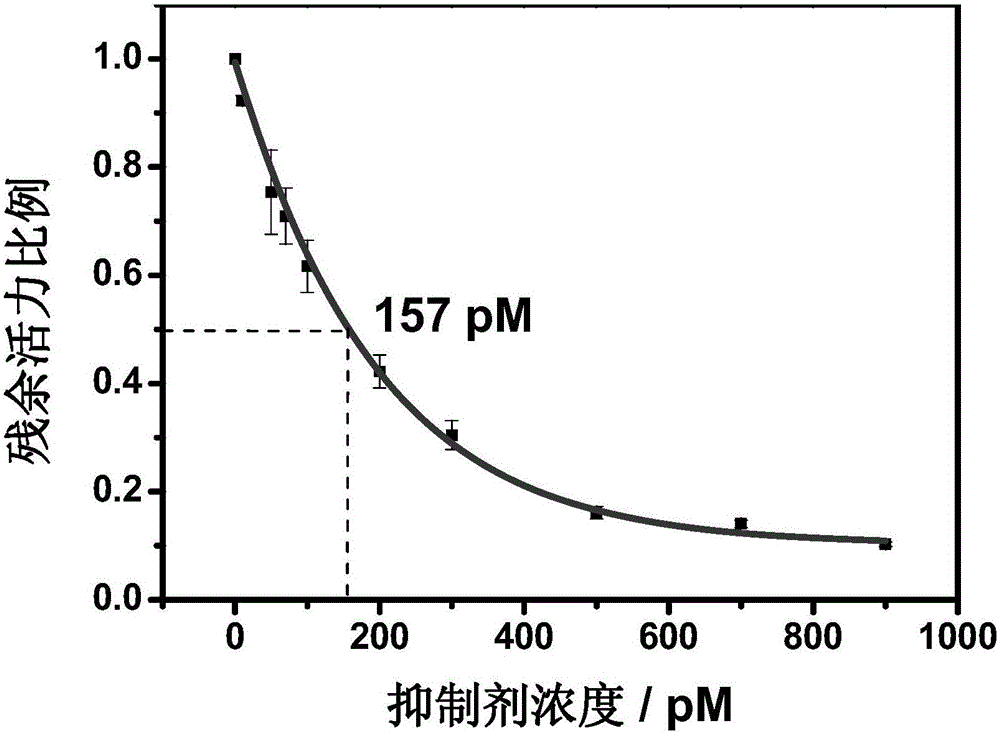
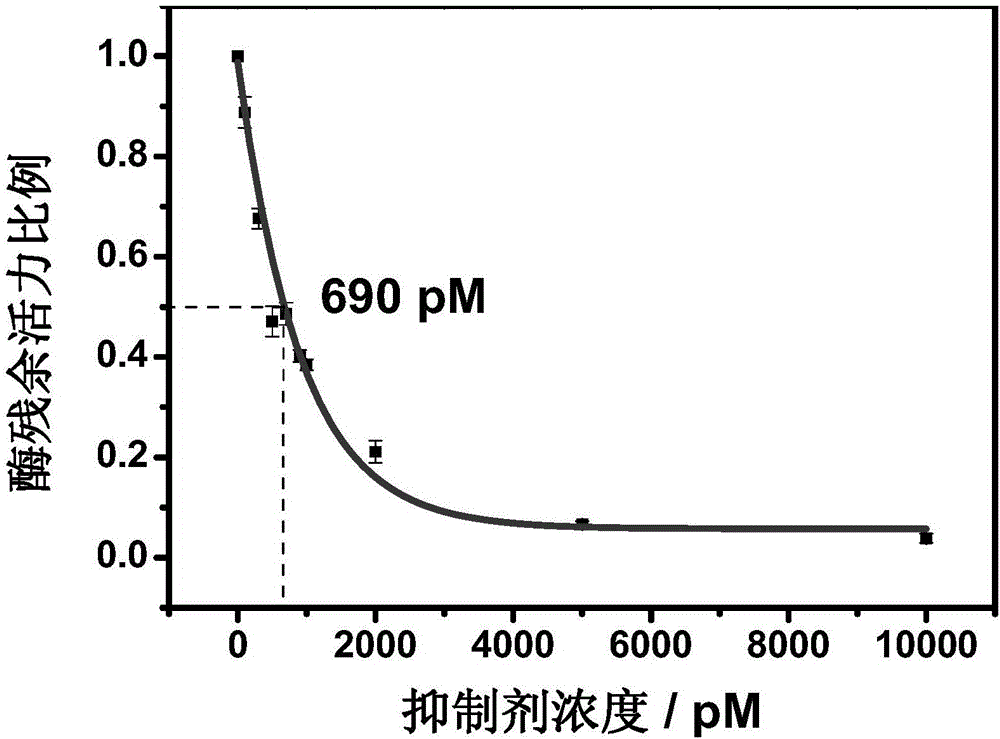
Nitrile cathepsin K inhibitors using trifluoroethylamino groups as P2-P3 linkers, and application of inhibitors
A protease inhibitor and cathepsin technology, which is applied in drug combination, organic chemistry, bone diseases, etc., can solve the problems of unfavorable improvement of inhibitor selectivity, consumption of inhibitor and enzyme binding energy, poor selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070]
[0071] First, 4'-bromo-2,2,2-trifluoroacetophenone (7.59g, 30mmol) and K 2 CO 3 (12.44g, 90mmol) was added to a round bottom flask (capacity 250mL), methanol (20mL) was added to dissolve, and then (6.51g, 36mmol) L-leucine methyl ester salt was added, stirred and refluxed under 50°C water bath for 16h. After this process is finished, the imine derivative b will be generated, and the reaction does not need to be processed again, and is directly used in the next step of the reaction.
[0072] Afterwards, a certain volume of anhydrous acetonitrile (100 mL) was added to the product system obtained in the first step, and the mixture was frozen and evacuated to remove oxygen, and then protected with nitrogen. Slowly add enough Zn(BH 4 ) 2 Tetrahydrofuran solution (2eq, about 0.5M×120mL), after the addition was completed, the reaction was maintained for 3h. After completion, add dilute hydrochloric acid to quench the reaction (add hydrochloric acid to no longer produc...
Embodiment 2
[0074]
[0075] Add c (5.52g, 15mmol) into a round bottom flask (capacity 100mL) filled with THF (20mL), and cool down to -25°C. Add N-methylmorpholine (2.341 mL, 18 mmol) followed by isobutyl chloroformate (1.832 mL, 18 mmol), and the solution will turn white. In addition, 1,2-dimethylhydrazine hydrochloride solution (2.66g, 20mmol, dissolved in 1mL water) and NaOH solution (5M, 8mL) were mixed under ice bath conditions, and the mixed solution was added to the above round bottom flask After 30 min, it was raised to room temperature, and then stirred at room temperature for 90 min. After evaporation to dryness, it was extracted with ethyl acetate (20 mL×4), and the organic phases were combined. Wash with saturated sodium bicarbonate, dilute hydrochloric acid, pure water successively, remove water with anhydrous magnesium sulfate, filter, and evaporate to dryness to obtain a white crude product, which is separated and purified by column chromatography to obtain 4.98g of com...
Embodiment 3
[0081]
[0082] Add d (4.10g, 10mmol) to a round bottom flask (capacity 100mL) filled with methanol (20mL), add sodium acetate (1.64g, 20mmol) and nitrile bromide (2.12g, 20mmol) to it, at room temperature Stir. After 3h, methanol was spin-dried, water (10mL) was added thereto, and KHSO 4 The pH of the solution was adjusted to 1-2. Then it was extracted four times with ethyl acetate (20 mL×4), and the organic phases were combined. The organic solvent was successively washed once with water, twice with saturated sodium bicarbonate and twice with saturated sodium chloride, and then dehydrated with anhydrous magnesium sulfate. After filtering and evaporating to dryness, a white crude product should be obtained, which was separated and purified by silica gel chromatography to obtain 3.27 g of compound 1' with a yield of about 75%.
[0083] Product H NMR, C NMR and mass spectrometry characterization:
[0084] 1 H NMR (500MHz, CDCl 3 ): δ7.51(d, J=8.2Hz, 2H), 7.30(d, J=8.5,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com