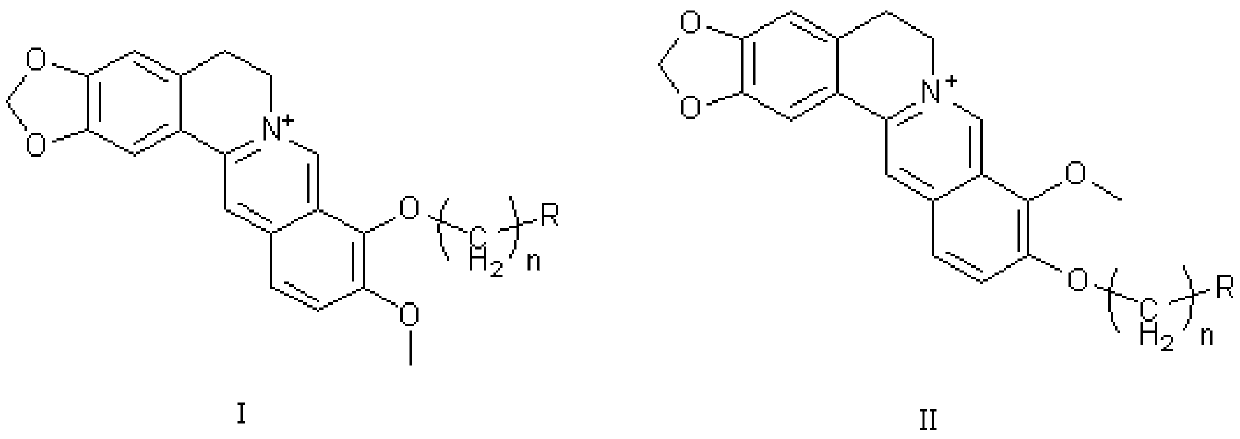
Application of berberine and its derivatives as hexosaminidase inhibitors
A technology of hexosaminidase and inhibitor, applied in the application field of inhibitors, can solve problems such as abnormal molting and death of insects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Taking hexosaminidase OfHex1 and HsHexB as targets, 35 compounds listed in Table 1 were screened for inhibitors. Specific steps are as follows:
[0029] Positive control: Set up 2 parallel positive controls. Under the conditions of 30℃ reaction temperature and 100μL reaction system, 2nmol / L glycosyl hydrolase and 50μmol / L substrate (MU-GlcNAc) were incubated in 20mmol / L pH 6.5 phosphate buffer for 30min, and then 100μL 0.5mol / L sodium carbonate solution terminated the reaction, and the reaction solution was excited with excitation light with a wavelength of 360nm, and then the absorbance value at a wavelength of 450nm was measured.
[0030] Experimental group: set up 3 parallel experimental groups. Under the conditions of 30°C reaction temperature and 100 μL reaction system, 2 nmol / L glycosyl hydrolase, 50 μmol / L substrate (MU-GlcNAc) and 10 μM compound were incubated in 20 mmol / L pH 6.5 phosphate buffer for 30 min, Afterwards, 100 μL of 0.5 mol / L sodium carbonate so...
Embodiment 2
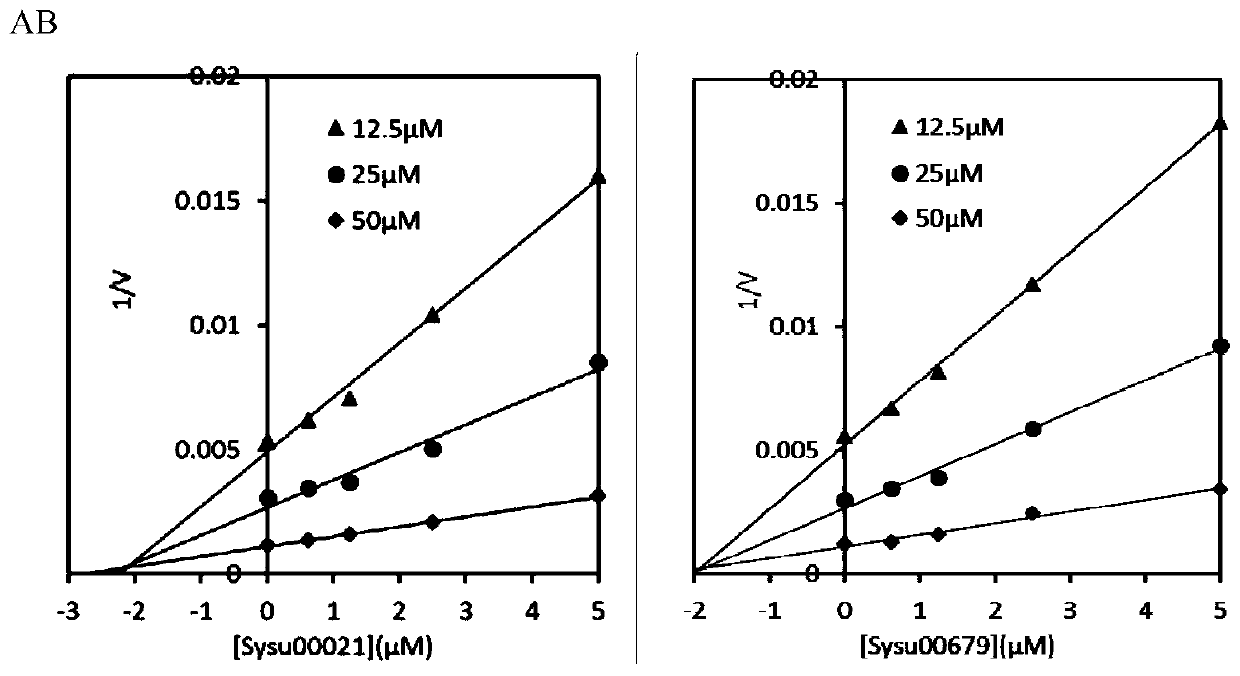
[0035]1) Inhibition constant K i determination
[0036] OfHex1: MU-GlcNAc was used as the substrate, and three groups of substrate concentration gradients were set up for the reaction, and the final concentrations were 12.5 μM, 25 μM and 50 μM, respectively. In each group of substrate concentrations, five groups of compound concentration gradients with final concentrations of 5, 2.5, 1.25, 0.625 and 0 μM were taken to measure the inhibitory activity. The reaction system is 100 μL, the buffer environment is 20 mM phosphate buffer, pH 6.5, the final enzyme concentration is 2 nM, the reaction temperature is 30 ° C, the reaction time is 30 min, and then 100 μ L of 0.5 M sodium carbonate solution is added to terminate the reaction, and the released MU After being excited by 360nm excitation light, the absorbance value was measured at a wavelength of 450nm. The data were plotted by Dixon method, and the inhibition constants Ki of Sysu-00021 and Sysu-00679 to OfHex1 were 2 μM and 1...
Embodiment 3
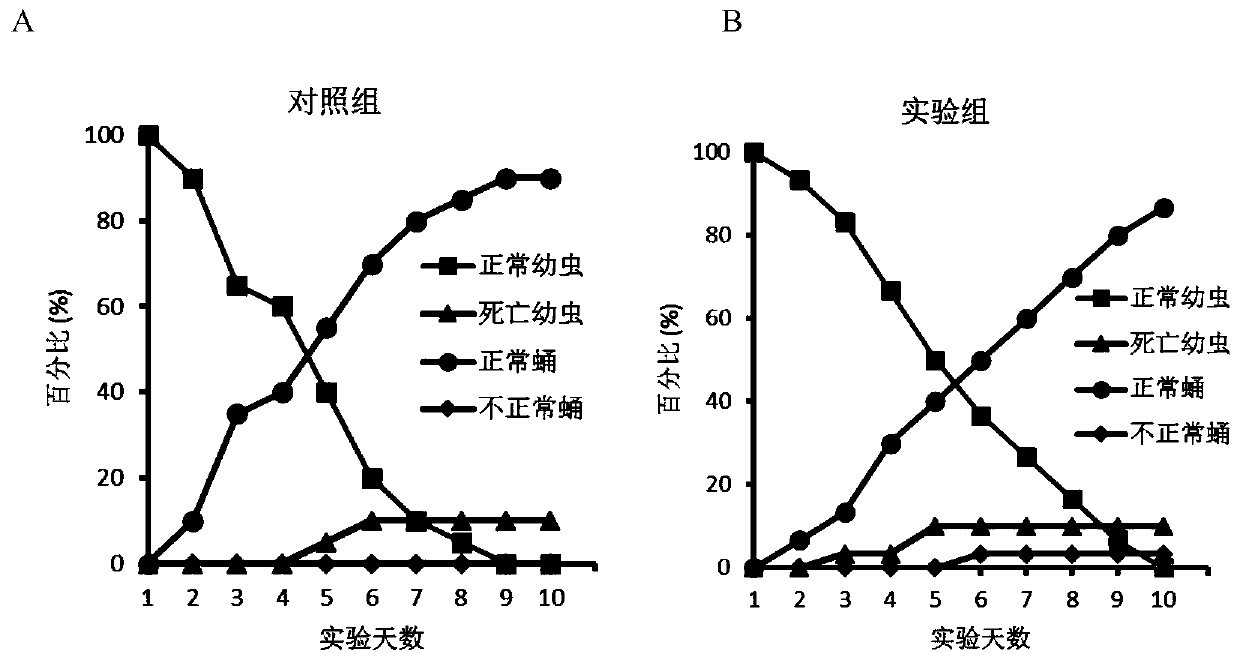
[0038] The specific steps for evaluating the insecticidal activity of the compound Sysu-00021 are as follows:
[0039] In the experiment, healthy larvae on the fourth day of the fifth instar were selected as experimental materials, and a control group and an experimental group were set up. The compound Sysu-00021 was dissolved in 5% DMSO, and the final concentration of the solution was 100 μM. The larvae of the control group were injected with 2 μL of 5% DMSO, and the larvae of the experimental group were injected with 2 μL of the compound Sysu-00021 at a concentration of 100 μM. The injected larvae were cultured at 26°C, with a relative humidity of 70%-90%, 16 hours of light per day, and 8 hours of darkness until all of them pupated. During this period, normal larvae, dead larvae, normal pupae and abnormal pupae Quantities and phenotypes were counted. The statistical results are plotted as figure 2 , where A is the control group and B is the experimental group. The resul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com