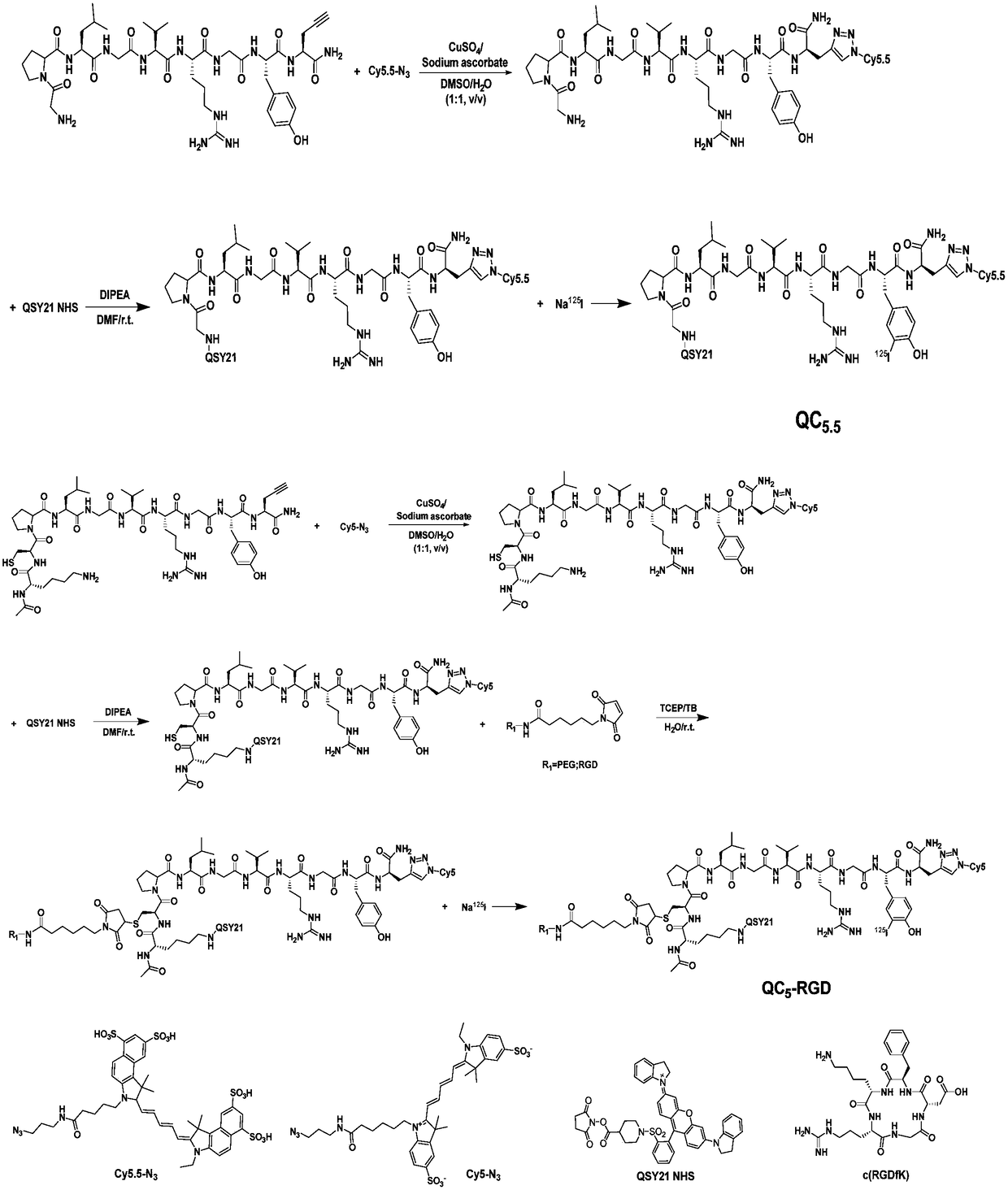
Matrix metalloproteinase-2 specific multi-modality molecular image probe and preparation method and application in preparation of tumor imaging agent thereof
A matrix metal and molecular imaging technology, applied in the directions of in vivo radioactive preparations, echo/ultrasonic imaging agents, general/multifunctional contrast agents, etc. performance, improve sensitivity and accuracy, and achieve the effect of precise positioning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Synthesis and characterization of matrix metalloproteinase-2 specific recognition multimodal molecular imaging probe
[0044] (1) Alkyne-terminated amino acid compound (GPL-Pra or KCP-Pra) (0.0055 mmol) and Cy5.5-N 3 or Cy5-N 3 (0.0050 mmol) was dissolved in 1 mL DMSO and stirred at room temperature to obtain a clear solution; sodium ascorbate (0.0060 mmol) and CuSO 4 (0.003 mmol) dissolved in 1 mL H 2 O, mixed evenly, added dropwise to the above DMSO solution, and stirred at room temperature in the dark for 3 h, and the reaction was monitored by HPLC. The solution after the reaction was separated and purified with a preparative chromatographic column to obtain the target product GPL-Cy5.5, HR-MS: C 85 h 115 N 19 o 23 S 4 , ([M+2H] 2+ ): 949.8726, found: ESI-MS: m / z 949.8612; KCP-Cy5, HR-MS: C 87 h 127 N 21 o 19 S 3 , ([M+H] + ): 1866.8857, found: ESI-MS: m / z 1866.7682.
[0045] (2) Dissolve GPL-Cy5.5 or KCP-Cy5 (0.005 mmol) and QSY21 NHS (0.00...
Embodiment 2
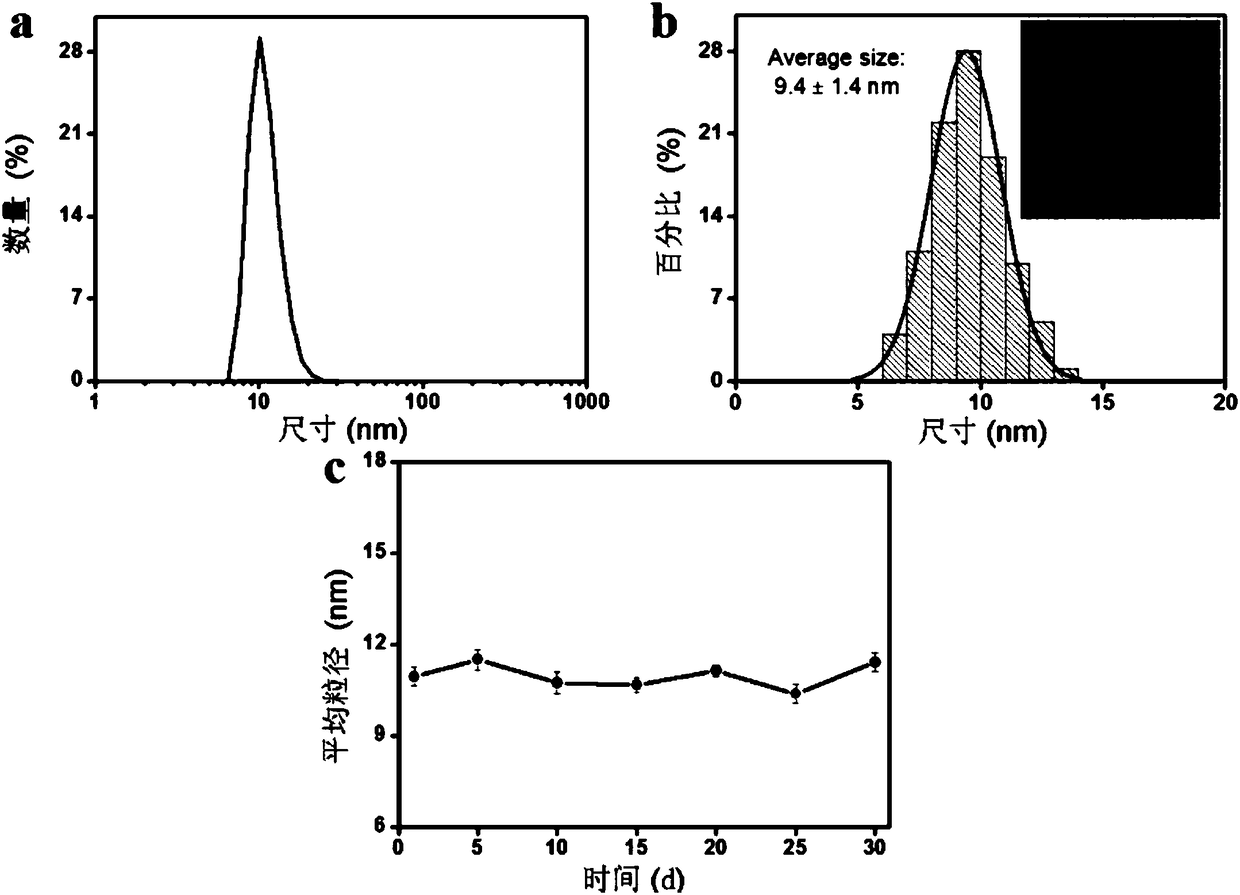
[0047] Example 2: Probe QC 5.5 Study on the hydrated particle size, TEM image and stability of
[0048] Prepare the purified probe QSY21-GPL-Cy5.5 into 40 μM PBS solution (pH = 7.4), and measure the hydrated particle size (see figure 2 a) and TEM (see figure 2 b). The solution is placed in a refrigerator at 4°C for 30 days, and the hydrated particle size is measured every 5 days. The data is as follows: figure 2 As shown in c, it shows that the probe has good stability in PBS; probe QC 5.5 (40μM) hydrated particle size in PBS (pH=7.4) (a); transmission electron microscopy and particle size statistics (b); stability at different times (c) see figure 2 .
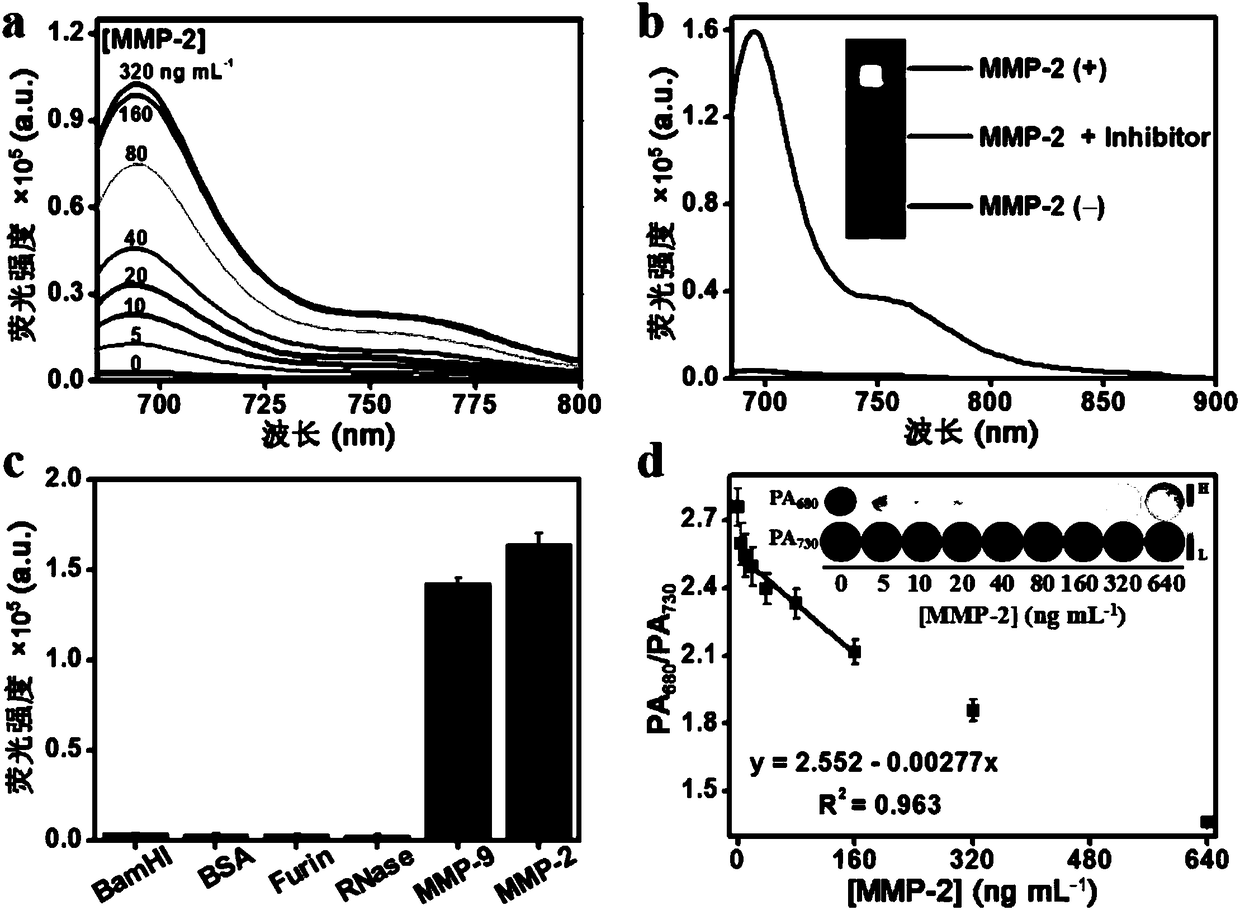
Embodiment 3
[0049] Example 3: Probe QC 5.5 Reaction studies with in vitro enzymes
[0050] Mix the probe (4 μM) with 0, 5, 10, 20, 40, 80, 160, 320 ng mL -1 Different concentrations of MMP-2 were added to the HEPES buffer solution (pH = 7.4), and after reacting at 37°C for 2 h, the fluorescence intensity of the solution after the reaction was detected by a FLS980 steady-state transient fluorescence spectrometer. image 3 Shown in a.
[0051] Mix the probe (4 μM) with MMP-2 (320 ng mL -1 ) into the HEPES buffer solution (pH = 7.4), reacted at 37°C for 2 h, and compared the changes before and after shearing with the probe that had not reacted with the enzyme by HPLC, as shown in image 3 Shown in b.
[0052] Mix MMP-2 inhibitor (GM6001, 100 μM) with MMP-2 (320 ng mL -1 ) at 37°C for 0.5 h, the probe (4 μM) was added, and the reaction was continued for 2 h. Probe (4 μM), probe (4 μM) and MMP-2 (320 ng mL -1 ) while reacting at 37°C for 2 h. The fluorescence intensity of the solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com