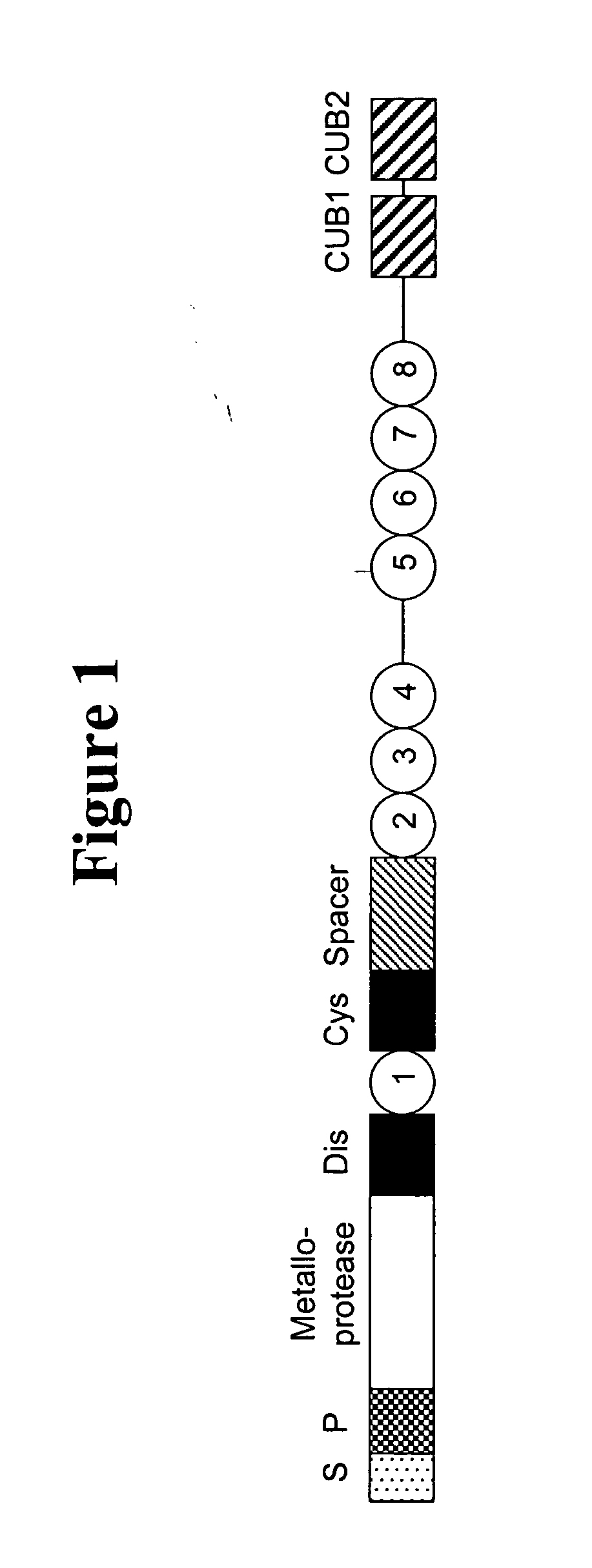
Methods and kits for measuring ADAMTS13/FXI complexes
a technology of adamts13 and complexes, which is applied in the field of adamts13 activity measurement and diagnosis of thrombocytopenia purpura, can solve the problems of affecting the clinical presentation of patients with ttp, the clinical manifestation of ttp is difficult to distinguish from hemolytic uremic syndrome, and the clinical manifestation of ttp is difficult to distinguish from ttp hemolytic syndrome, so as to reduce the amount of adam
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Measurement of ADAMTS13 Activity in PRP Using LVY-pNA and LVY-AMC Peptidyl Substrates
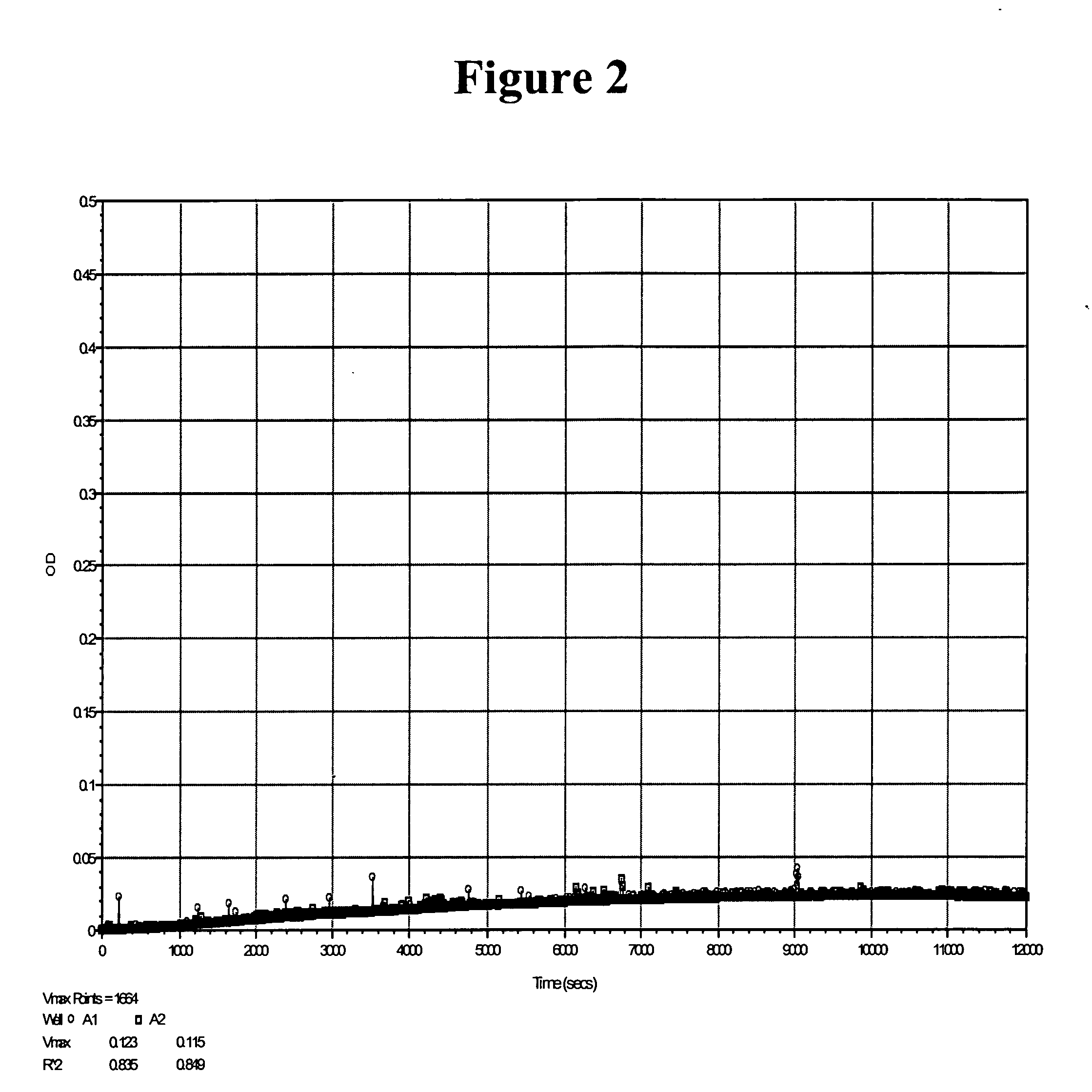
[0114] As discussed above, Furlan et al. reported that some peptidyl-pNA moieties, such as XLY-pNA and XVY-pNA, were not substrates for ADAMTS13. We prepared LVY-pNA and tested it for its ability to be cleaved by ADAMTS13. The results shown in FIG. 2 confirm the findings of Furlan et al., i.e., LVY-pNA was not cleaved by ADAMTS13.
[0115] Thus, the nature of the leaving group of the peptidyl substrate appears to be important in determining the ability of ADAMTS13 to cleave a chromophor or a fluorophor from the end of a peptidyl substrate.
example 2
Measurement of ADAMTS13 Activity in PRP Using Peptidyl Substrates of Different Lengths
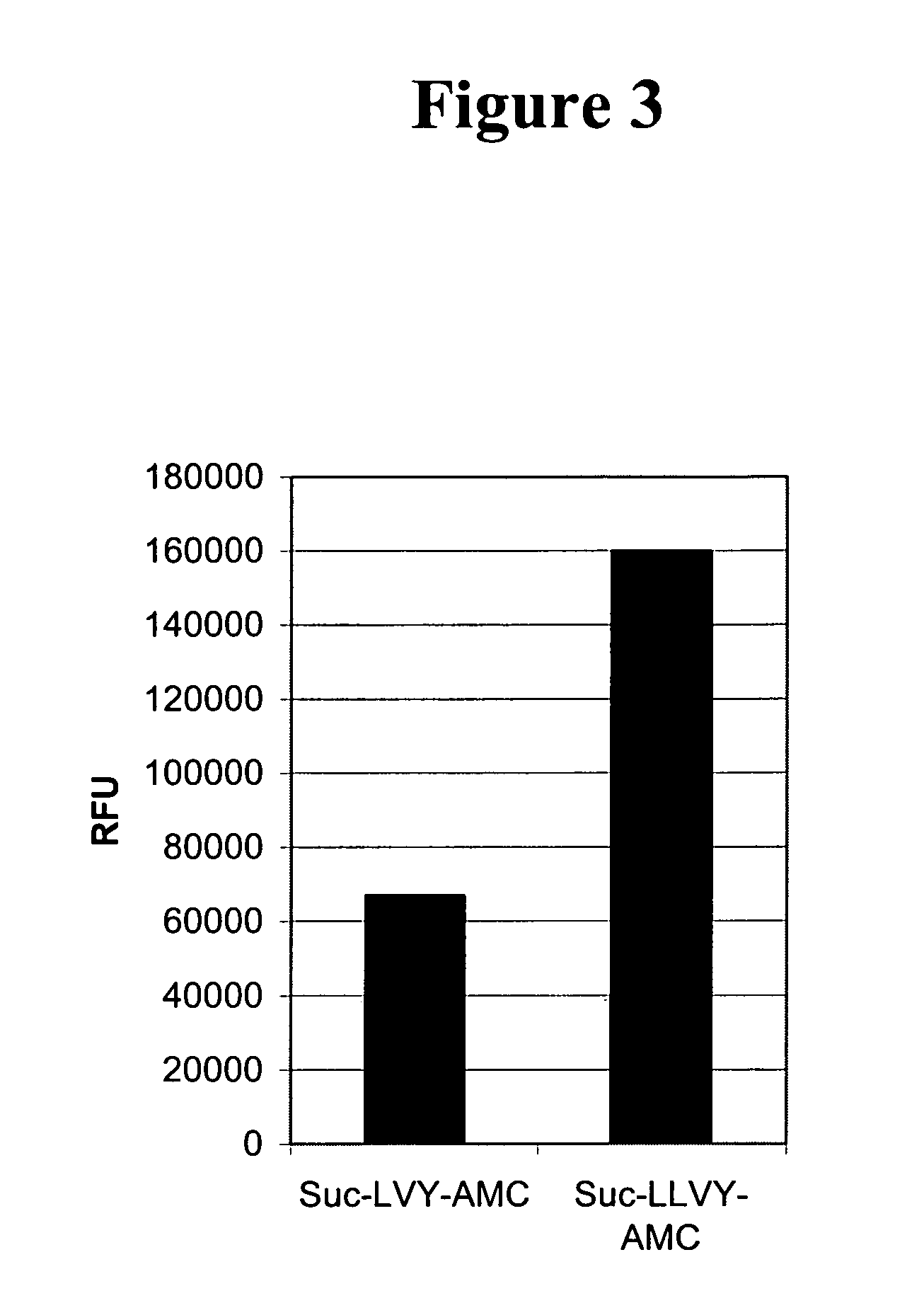
[0116] In order to determine the effect of modifying the length of the peptide sequence conjugated to the AMC fluorochrome, normal PRP (20 μl) was incubated at 37° C. with a final concentration of 0.8 mM Suc-LLVY-AMC or LVY-AMC in 10 mM Tris-HCl pH 8.0 buffer in total volume of 200 μl. Fluorescence was measured using a microtiter fluorophotometric plate reader (Ex 360 nm / Em 460 nm). Both peptidyl-AMC substrates were cleaved by ADAMTS13 in PRP, with the Suc-LLVY-AMC giving a higher signal over time as compared to the LVY-AMC substrate (FIG. 3).
[0117] This example demonstrates that different ADAMTS13 substrates can be made by altering the peptide sequence that is conjugated to the AMC fluorophor.
example 3
Measurement of ADAMTS13 Activity Using a FRET Substrate
[0118] ADAMTS13 activity was measured using a fluorescent substrate attached to a donor moiety and an acceptor moiety that functions by fluorescence resonance energy transfer (FRET). The ADAMTS13 selective substrate, NH2-Arg-Lys(DABCYL)-NLVYMVTGD(EDANS)-Arg-COOH, was used in this example. The NLVYMVTGD peptide sequence of this substrate is homologous to the ADAMTS13 cleavage site on VWF. The intact peptidyl substrate has low fluorescence, due to quenching of the DABCYL-fluorochrome by the EDANS-quencher. Cleavage of the substrate between the tyrosine and methionine of the peptide sequence results in an increase in fluorescence.
[0119] Isolated platelets in 10 mM Tris-HCl pH 8.0 assay buffer were incubated with a final concentration of 8 μg / ml of the FRET substrate at 37° C. The fluorescence was measured in a spectrofluorometric plate reader (Ex 360 nm / Em 440 nm). FIG. 4 shows that incubation of platelets with NH2-Arg-Lys(DABCYL...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrophoretic analysis | aaaaa | aaaaa |
| optical density | aaaaa | aaaaa |
| fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com