Antibody specifically binding to novel coronavirus
A virus and antibody technology, applied in the direction of virus/phage, antibody, virus, etc., can solve the problem of little knowledge about the potential therapeutic application of combined antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Example 1: Expression of SARS-COV-2 viral N protein
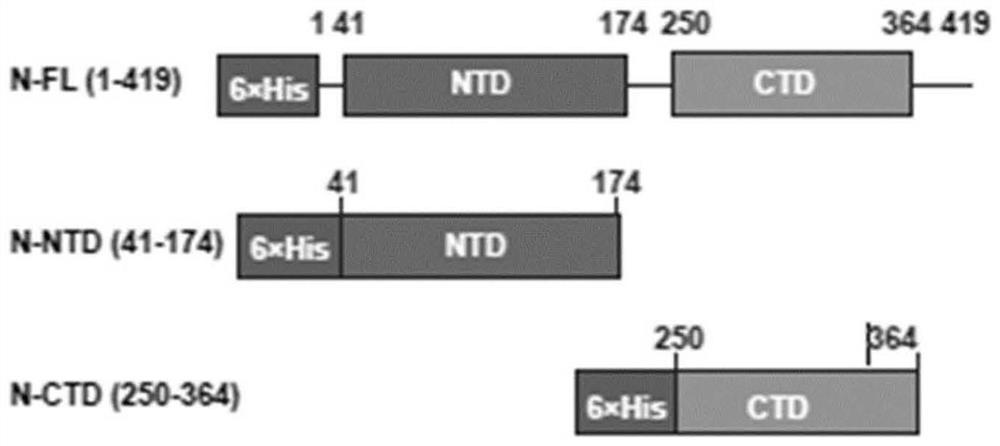
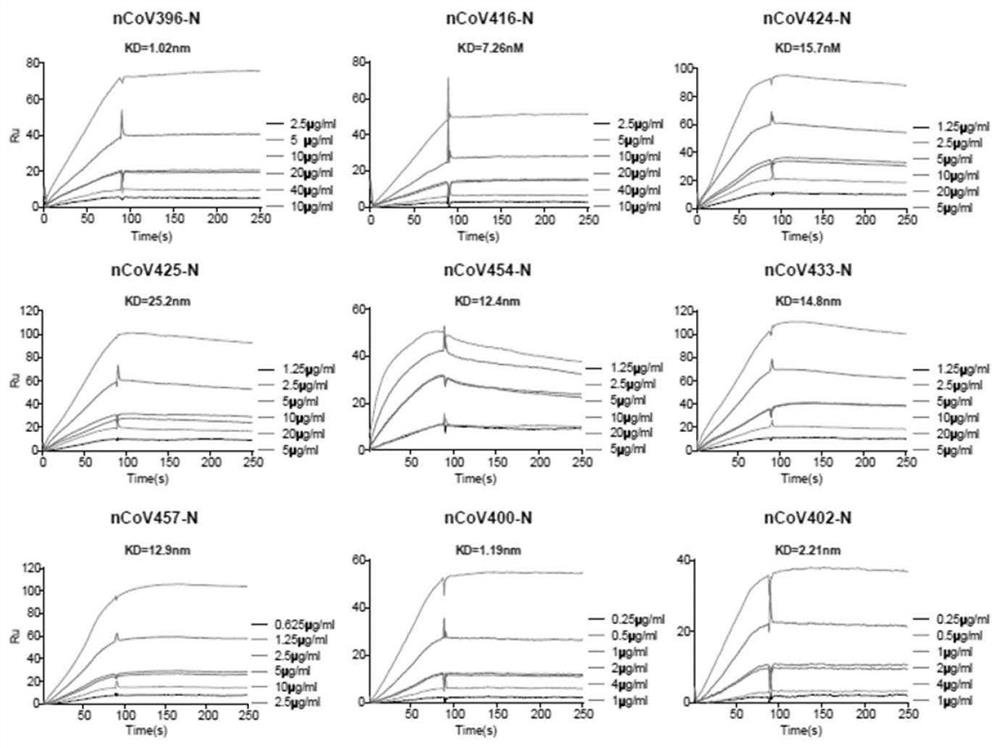
[0112] Sequence information (NCBI ID YP_009724397.2) (NCBI ID YP_009724397.2) is obtained from the public data, and the expression of N protein (full length) is synthesized: N-NFL (1-419), N-NTD ( N protein N end, 41-174) and N-CTD (N protein C end, 250-364), for example, figure 1 Indicated. The expression carrier is constructed according to conventional methods to convert suitable host cells to carry out corresponding expression. Briefly, 0.1 mM IPTG was added to the culture medium of the host cell containing the expression vector, and oscillated induced by oscillating culture at 25 ° C, 220 RMP, induced overnight (15h), induced protein expression. The expression of the protein was harvested and the purified protein was isolated by nickel pillars and was subjected to SDS-PAGE electrophoresis. The correct target protein (N protein) was used to screen anti-SARS-COV-2 viral N protein from blood cells from COVID-19 rehabili...
Embodiment 2
[0113] Example 2: Subselect memory B cell
[0114] According to relevant laws and regulations, the 6 volunteers were recruited from the Fifth Affiliated Hospital of Sun Yat-sen University, and the clinical rehabilitation of two throat swab new coronavirus nucleic acid detection is negative, 9-25 days after the onset, collect them Peripheral blood, separated monocytes (PBMC), for example, please refer to the invention patents of the authorization notice number CN107760690B. Serum antibody titer titer detection (ELISA) was performed using purified N protein as an antigen.
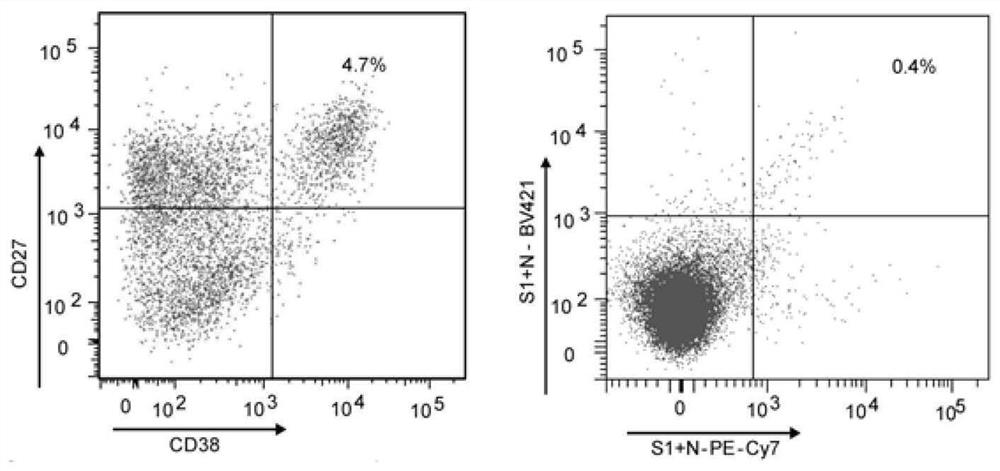
[0115] According to the sample antibody titer determined by the ELISA, the highest antibody titer (which is 1: 328050, which is 1: 328050), and a single slurry cell by flow cytometer (BD FACS ARIA III), with CD3 - / CD14- / CD16- / CD235A- / CD19 + / CD20LOW-NEG / CD27HI and CD38HI (BD Biosciences and Invitrogen) set single slurry cells, with CD3- / CD14- / CD16- / CD235A- / CD20- / CD19 + / CD27 + / SARS-COV2 S ...
Embodiment 3
[0116] Example 3: Separation, identification of the variable region gene of the antibody
[0117] Referring to the invention of an invention patent, a suitable primer is designed, and the variable region gene obtained by the screening obtained is amplified, and the corresponding antibody gene sequence is obtained. The specific operation is:
[0118] 1. Anti-transcription synthesis cDNA first chain
[0119] To the 96-well plate containing a single B cell, a constant region primer and a SUPERSCRIPT IV reverse transcriptase (InvitroT IV reverse transcriptase) were added to the light chain, respectively, 37 ° C for 1 hour in 96-well plates containing a single B cell.
[0120] 2. Separate antibody gene by two-wheel PCR program
[0121] The first round of PCR: 50 ul system contains 5 ul of reverse transcription reaction products, 25 μl of TAQ MIX (Invitrogen, Carlsbad, Ca), and 0.5 um of each sub-heavy chain and light chain antibody constant region primers, PCR reaction conditions: The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com