Quantitation of individual protein kinase activity
a technology quantitation, applied in the field of individual protein kinase activity, can solve the problems of extended assay completion time, inability to produce similar antibodies to phosphoserine and phosphothreonine, and limited detection of tyrosine kinases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Kinase Activity of cAMP-Dependent Protein Kinase
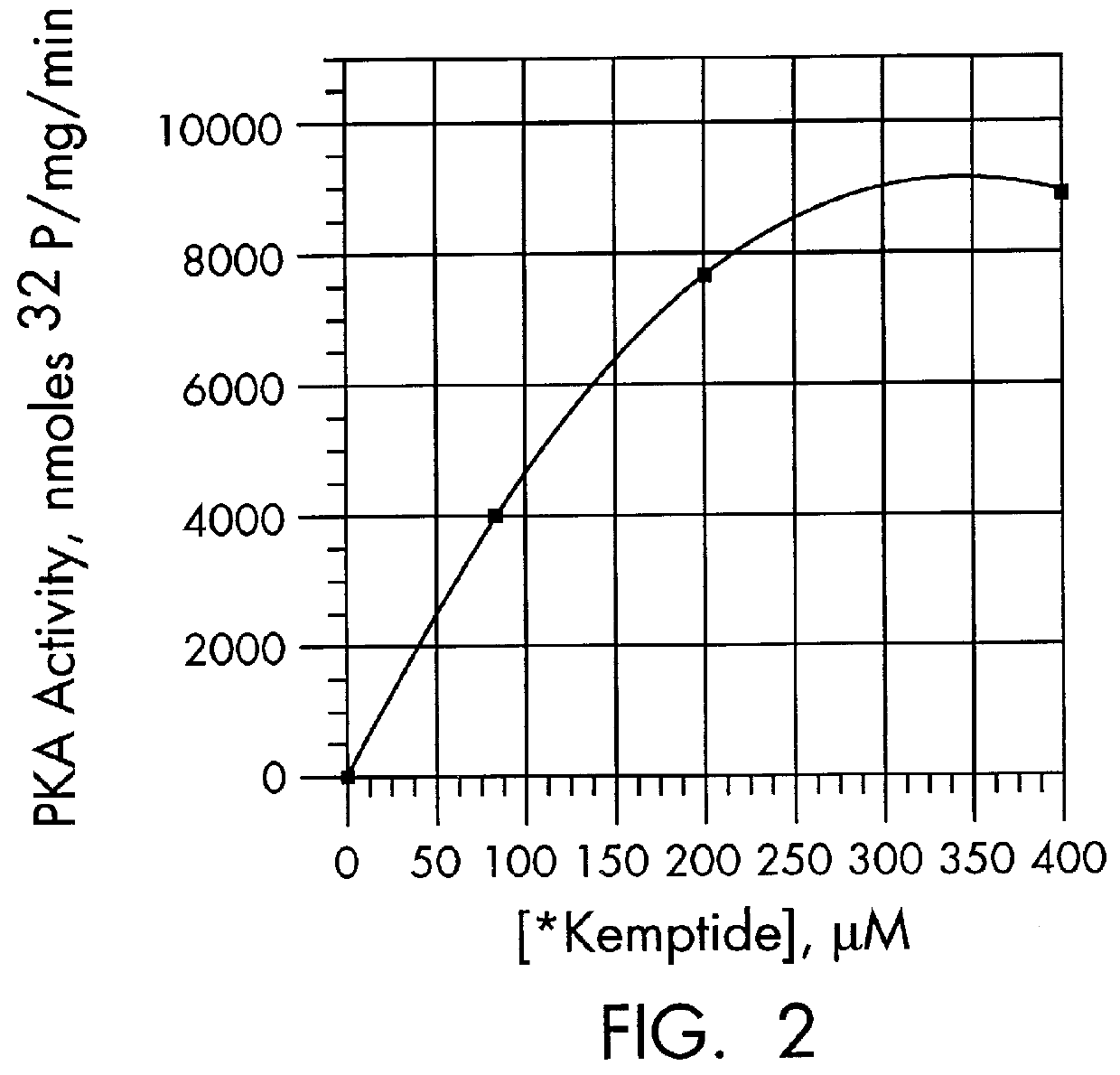
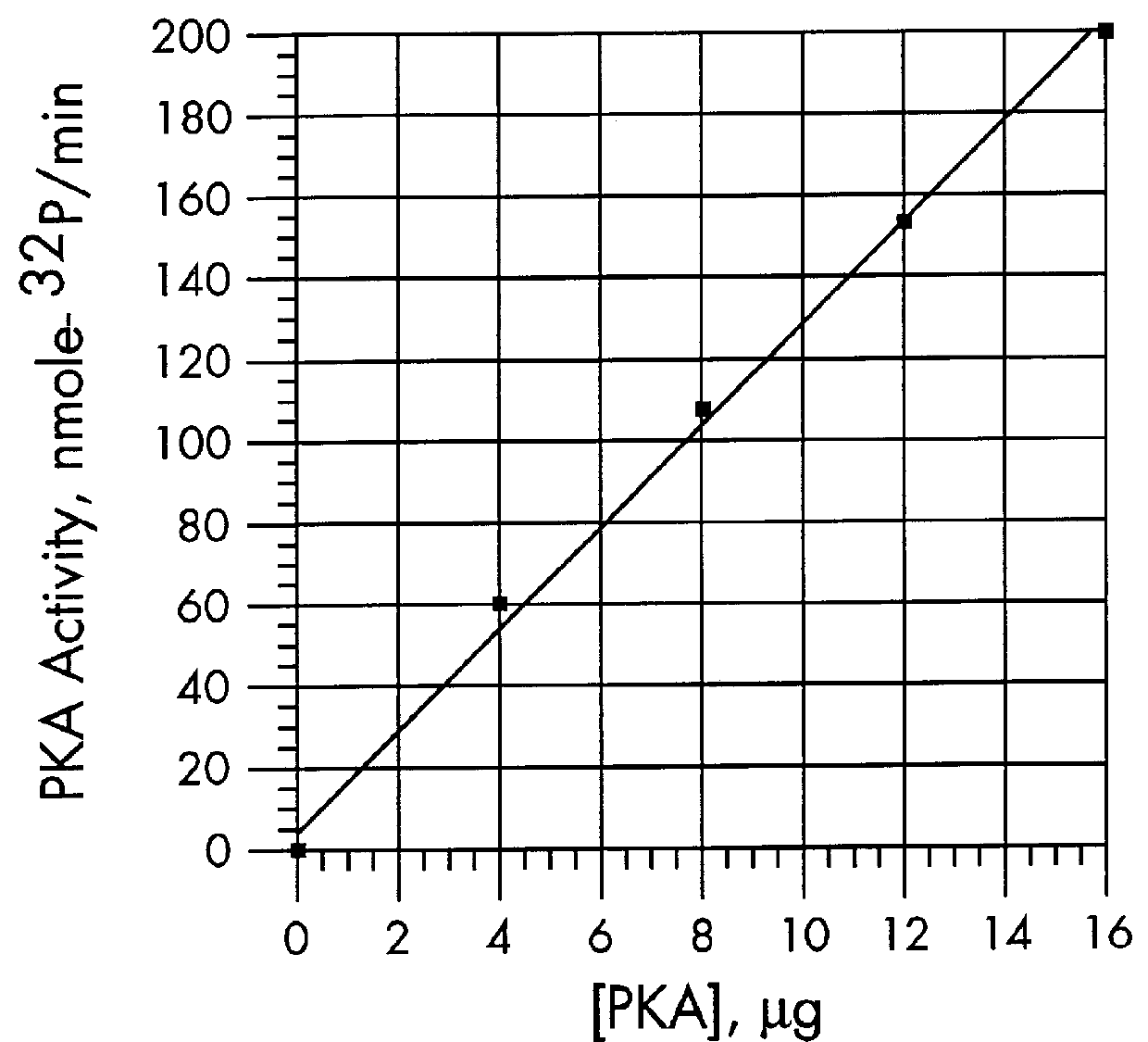
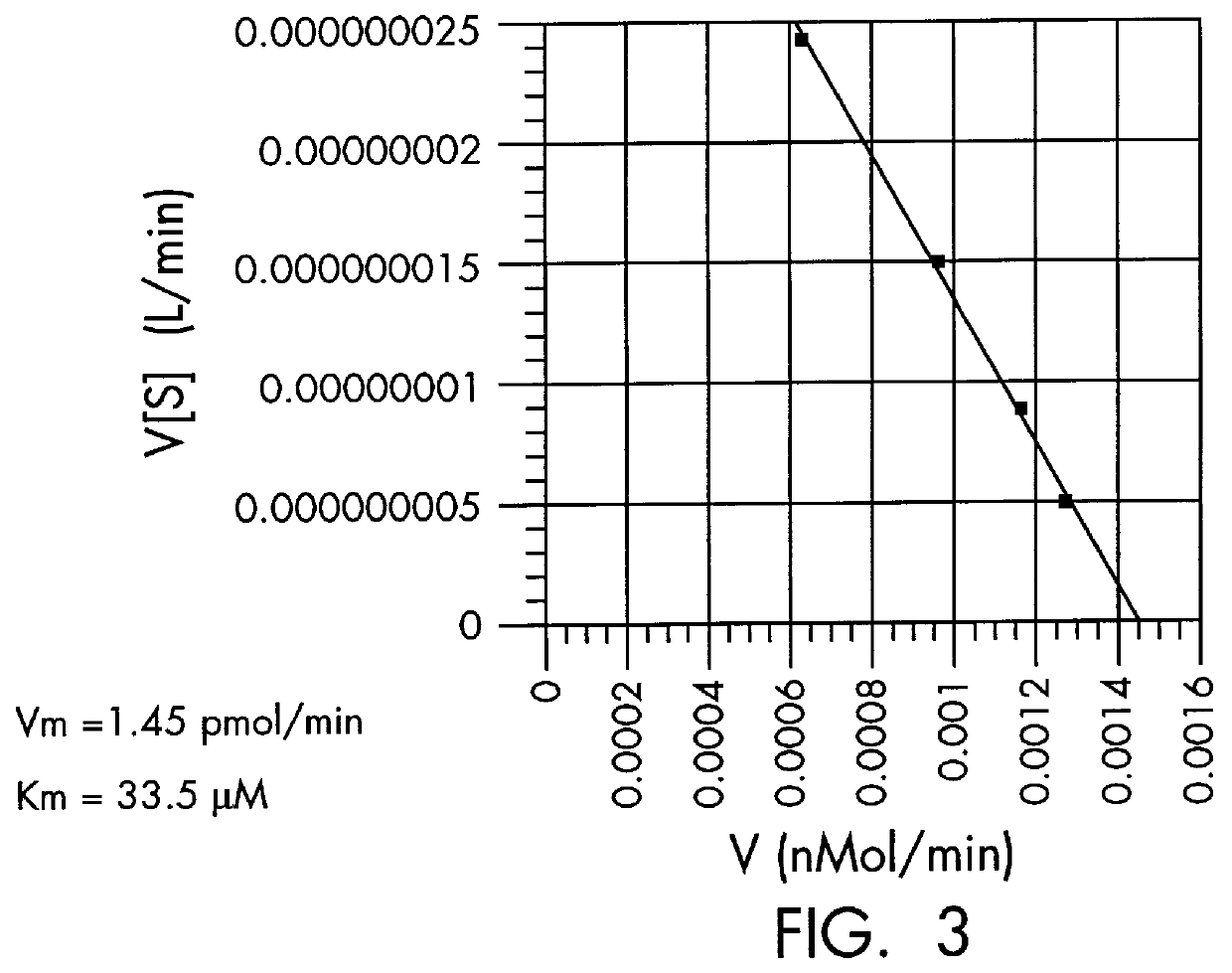
The kinase activity of cAMP-dependent protein kinase (PKA) was carried out in duplicates at each of the various peptide substrate concentrations, in a reaction volume (50 .mu.L) containing 40 mM Tris HCl, pH 7.4, 20 mM MgCl.sub.2, 100 .mu.M .gamma.-.sup.32 P-ATP (sp.act. of 100-200 cpm / pmol), 100 .mu.g / ml of bovine serum albumin (BSA), and the appropriate biotinylated peptide substrate: *-Leu-Arg-Arg-Ala-Ser-Leu-Gly (Promega Peptide A, SEQ. ID. NO: 1, M.Wt of 998.4).
The reaction was started by addition of the appropriate amount of enzyme (Promega #V5221) to give 2 Kemptide units and incubated for 0, 5, and 10 minutes at 37.degree. C. (one Kemptide unit is defined as the amount of enzyme required to catalyze the incorporation of 1 pmol of phosphate into Kemptide substrate in 1 minute). At specified periods of time at the appropriate temperature, the reaction was terminated with the addition of 10 .mu.L of terminating solution as describ...
example 2
Kinase Activity of PKA in Rat Tissues
The kinase activity of PKA in various rat tissues was also investigated using the modified peptide substrate and assay protocol as described above.
As illustrated in FIG. 5, the protein kinase activity of PKA was 2.2, 1.75, 3.2, and 1.9 nmol .sup.32 P / min / mg of rat liver, ovary, heart, and brain, respectively, and was comparable to those obtained with unmodified peptide and the phosphocellulose paper method. The phosphate incorporation into the modified substrate was completely abolished when 100 .mu.M of PKA inhibitor (PKI) was included in the reaction. Thus, the phosphorylation of the modified peptide substrate, under the conditions described above and assayed with the protocol, is catalyzed by PKA and not by other protein kinases present in the tissue extract.
Any protein(s) that is phosphorylated in the reaction and has a basic charge will not bind to the matrix. Only the phosphorylated modified peptide substrate is bound to the matrix. Thus, t...
example 3
Protein Kinase Activity of Ca.sup.2+ and Phospholipid Dependent Protein Kinase
The protein kinase activity of Ca.sup.2+ and phospholipid dependent protein kinase activity (PKC) was determined in a similar manner to that of PKA described above, except that the biotinylated peptide substrate was a derivative of neurogranin; namely, biotinylated neurogranin .sub.(28-43) Chen, S-J, et al., 1993.
The peptide was modified by the addition of biotin containing moiety: *-Ala-Ala-Lys-Ile-Gln-Ala-Ser-Phe-Arg-Gly-His-Met-Ala-Arg-Lys-Lys (Promega Peptide B, SEQ. ID. NO: 2, Mol.Wt. is 2139.6), and was used at a concentration of 100 .mu.M. The enzyme (#V5261, Promega Corporation) was diluted 5.times. in 100 .mu.g / ml of BSA.
The kinase activity of the PKA was determined in a reaction volume of 50 .mu.L consisting of 40 mM Tris. HCl, pH 7.4, 10 mM MgCl.sub.2, BSA at 100 .mu.g / ml, and 100 .mu.M of .gamma.-.sup.32 P-ATP (sp.act. of 100 cpm / pmol). The activity was determined in the presence and absence of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Radioactivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com