Method for preparing monoclonal antibody of spike protein of novel coronavirus
A monoclonal antibody and spike protein technology, applied in antiviral immunoglobulin, virus/phage, biochemical equipment and methods, etc., can solve the problem of no effective drugs, reduce the cure rate of SARS-CoV-2 virus, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]A preparation method of a novel coronavirus spike protein monoclonal antibody, the specific steps are as follows:
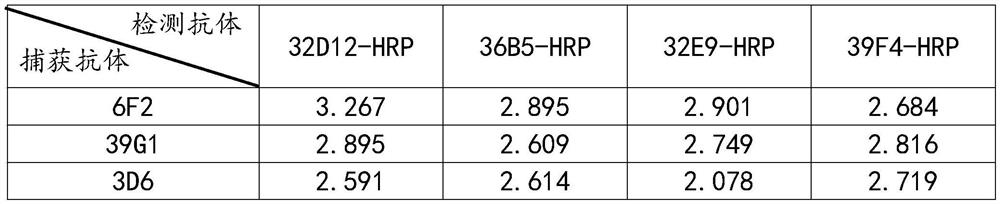
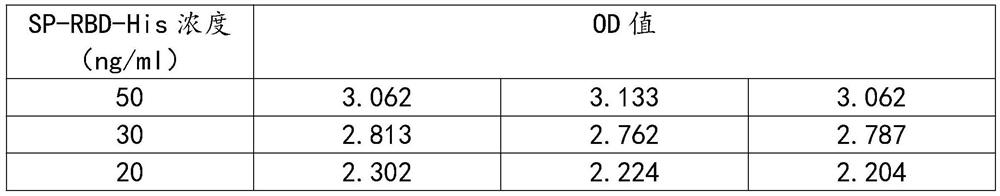
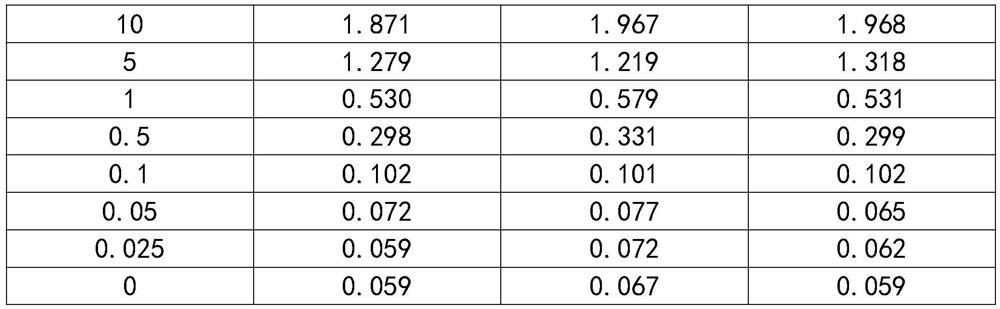
[0025]1 Antigen preparation: Complete gene synthesis of Skipe protein RBD region sequence, subcloned into eukaryotic expression vector, and add 8*His tag to C-terminal of expression vector to express purified spike protein receptor binding domain SP-RBD -His;
[0026]2 Animal immunization: Balb / c mice were immunized with spike protein receptor binding domain SP-RBD-His, each Balb / c mouse was immunized with 50 μg each time, immunized 3 times, each time interval was two weeks;
[0027]3 Cell fusion: Take the mouse spleen 1 week after three immunizations, put the mouse spleen into a petri dish containing 5mL DMEM solution, crush the spleen, filter with nylon mesh to obtain a single cell suspension, and suspend the single cell The liquid and logarithmic phase mouse myeloma cell SP2 / 0 cell liquid were mixed at a volume ratio of 1:1, and the cell fusion was performed with a cel...
Embodiment 2
[0032]A preparation method of a novel coronavirus spike protein monoclonal antibody, the specific steps are as follows:
[0033]1 Antigen preparation: Complete gene synthesis of Skipe protein RBD region sequence, subcloned into eukaryotic expression vector, and add 8*His tag to C-terminal of expression vector to express purified spike protein receptor binding domain SP-RBD -His;
[0034]2 Animal immunization: Balb / c mice were immunized with spike protein receptor binding domain SP-RBD-His, each Balb / c mouse was immunized with 50 μg each time, immunized 3 times, each time interval was two weeks;
[0035]3 Cell fusion: Take the mouse spleen 1 week after three immunizations, put the mouse spleen into a petri dish containing 5mL DMEM solution, crush the spleen, filter with nylon mesh to obtain a single cell suspension, and suspend the single cell The liquid and logarithmic phase mouse myeloma cell SP2 / 0 cell liquid were mixed at a volume ratio of 1:1, and the cell fusion was performed with a cel...
Embodiment 3
[0040]A preparation method of a novel coronavirus spike protein monoclonal antibody, the specific steps are as follows:
[0041]1 Antigen preparation: Complete gene synthesis of Skipe protein RBD region sequence, subcloned into eukaryotic expression vector, and add 8*His tag to C-terminal of expression vector to express purified spike protein receptor binding domain SP-RBD -His;
[0042]2 Animal immunization: Balb / c mice were immunized with spike protein receptor binding domain SP-RBD-His, each Balb / c mouse was immunized with 50 μg each time, immunized 3 times, each time interval was two weeks;
[0043]3 Cell fusion: Take the mouse spleen 1 week after three immunizations, put the mouse spleen into a petri dish containing 5mL DMEM solution, crush the spleen, filter with nylon mesh to obtain a single cell suspension, and suspend the single cell The liquid and logarithmic phase mouse myeloma cell SP2 / 0 cell liquid were mixed at a volume ratio of 1:1, and the cell fusion was performed with a cel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com