Method for purifying adalimumab by aid of cation exchange chromatography
A cation exchange and adalimumab technology, which is applied in the field of cation exchange chromatography purification of adalimumab, can solve the problems of inconvenient removal of polymers, denaturation and inactivation of antibodies, and high concentration of additives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0026] The specific embodiment of the present invention comprises the following steps:
[0027] 1) After fermenting and expressing CHO cells, separate the bacterial liquid;
[0028] 2) Dilute the fermentation broth with water, and adjust the pH to 6.00±0.10;
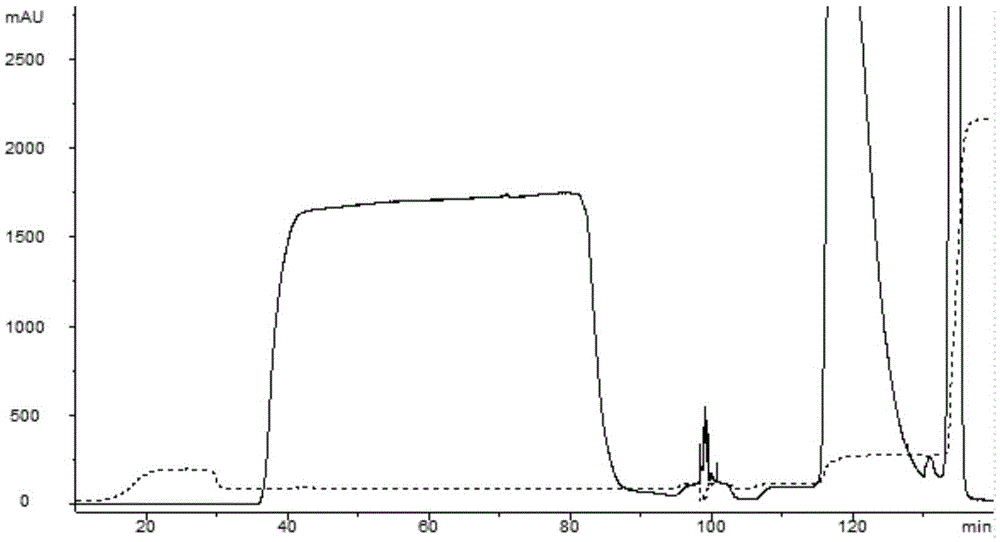
[0029] 3) Pass through a cation exchange column, use a buffer solution with a pH of 5.5-6.5 containing 0.1-0.5 mol / L urea for the first elution, and then use a beet with a pH of 5.5-6.5 and a mass fraction of 0.1%-1.0% The buffer solution of the alkaline surfactant is eluted for the second time, and the eluted components are collected;
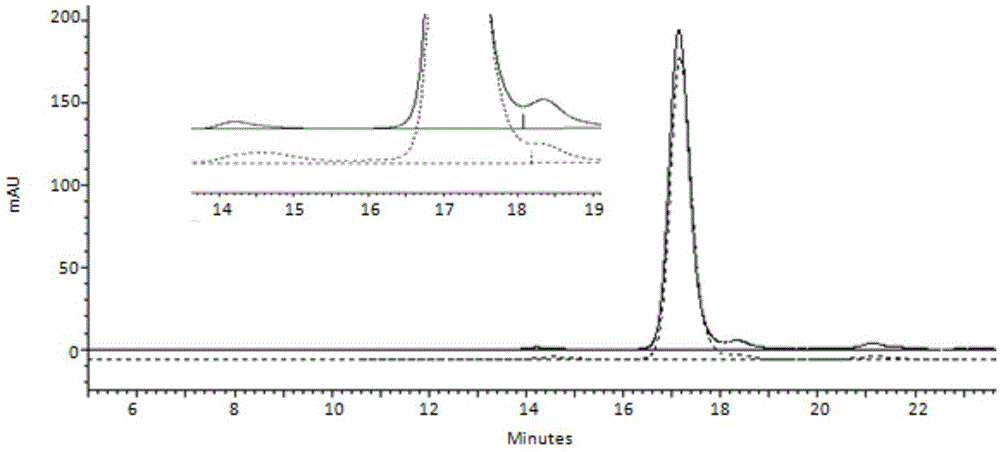
[0030] 4) The collected components are eluted by hydrophobic interaction chromatography, and the eluted components are collected.
[0031] In some embodiments, the betaine surfactant is selected from dodecyl dihydroxyethyl betaine, octadecyl dihydroxyethyl betaine or cocamidopropyl betaine.
[0032] In some embodiments, the separation in step 1) is to centrifuge the fermentation broth...
Embodiment 1
[0044] 1) Fermentation broth acquisition
[0045] CHO cells (Chinese hamster ovary cells) expressing fully human adalimumab were cultured and expressed adalimumab, and the expressed antibody was centrifuged at 4000-8000g for 10-15min on a SIGMA6K15 centrifuge to separate cells and For the fermentation broth, the supernatant was centrifuged for the second time, centrifuged at 6000-8000g for 10-15min to remove cell debris, and the supernatant was transferred to the next step.
[0046] Filtration: The supernatant after twice centrifugation is filtered through a 0.45μm filter membrane, and the filtered feed liquid can be directly loaded on the cationic column after adjustment.
[0047] 2) Cation exchange column (CEX)
[0048] 1) Sample adjustment: Dilute the clarified feed solution by 1 time, adjust the pH to 6.0 with 0.5M citric acid, and the volume is about 210ml at this time;
[0049] 2) Equilibration: AKTA purifer, a chromatography system of GE Company, is used, and an XK16 / ...
Embodiment 2
[0070] 1) Fermentation broth acquisition
[0071] CHO cells (Chinese hamster ovary cells) expressing fully human adalimumab were cultured and expressed adalimumab, and the expressed antibody was centrifuged at 4000-8000g for 10-15min on a SIGMA6K15 centrifuge to separate cells and For the fermentation broth, the supernatant was centrifuged for the second time, centrifuged at 6000-8000g for 10-15min to remove cell debris, and the supernatant was transferred to the next step.
[0072] Filtration: The supernatant after twice centrifugation is filtered through a 0.45μm filter membrane, and the filtered feed liquid can be directly loaded on the cationic column after adjustment.
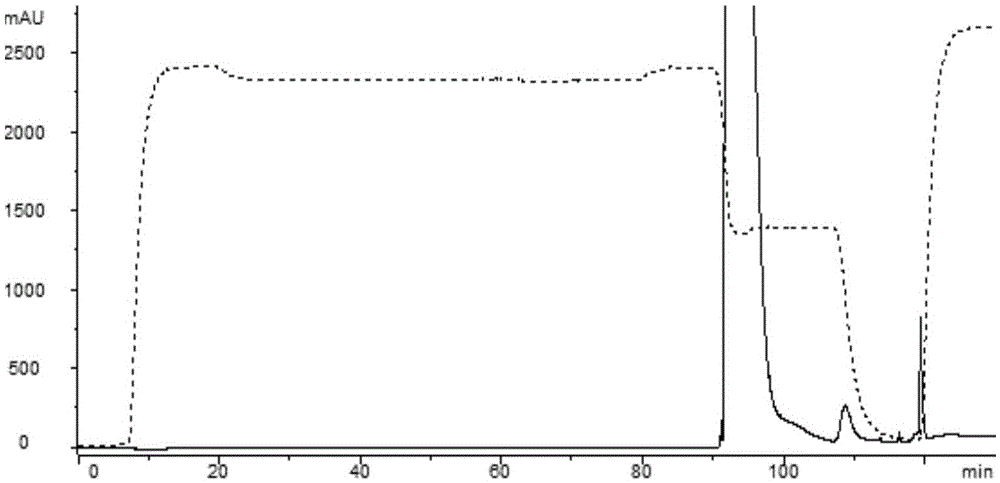
[0073] 2) Cation exchange column (CEX)
[0074] 1) Sample adjustment: Dilute the clarified feed solution by 1 time, adjust the pH to 6.0 with 0.5M citric acid, and the volume is about 400ml at this time;
[0075] 2) Equilibration: AKTA purifer, a chromatography system of GE Company, is used, and an XK16 / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com