Clinical effect of pharmaceutical products using communication tool integrated with compound of several pharmaceutical products
a communication tool and technology for pharmaceutical products, applied in the field of improving the usage and clinical efficacy of pharmaceutical products, can solve the problems of no verification, clinical trials, and many patients forced to emergency healthcare visits, so as to improve the clinical effect, improve the safety and quality of life, and improve the clinical effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0220]Study 1
[0221]A study is to be performed according to the following description in order to exemplify and show the clinical effect of the invention.
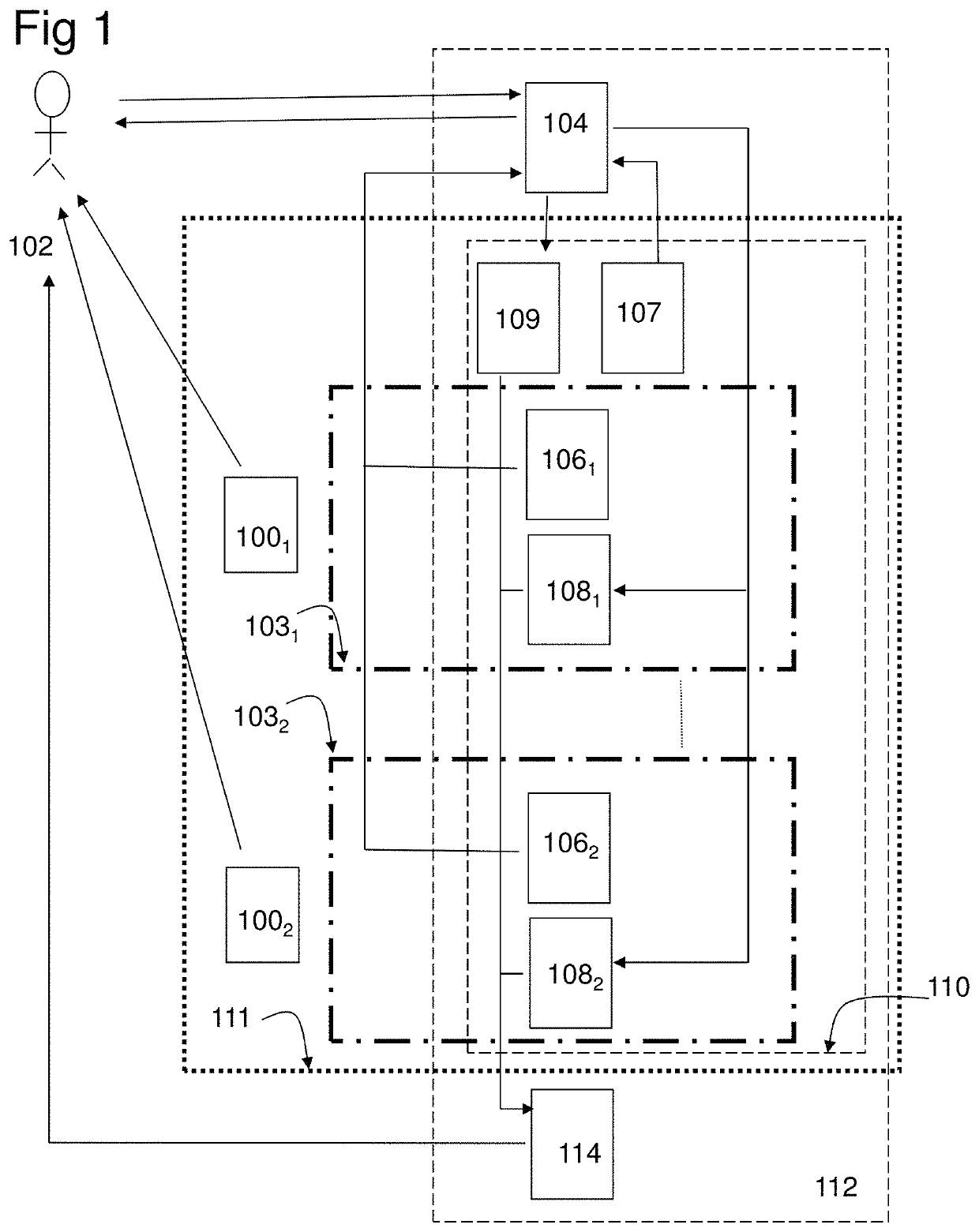
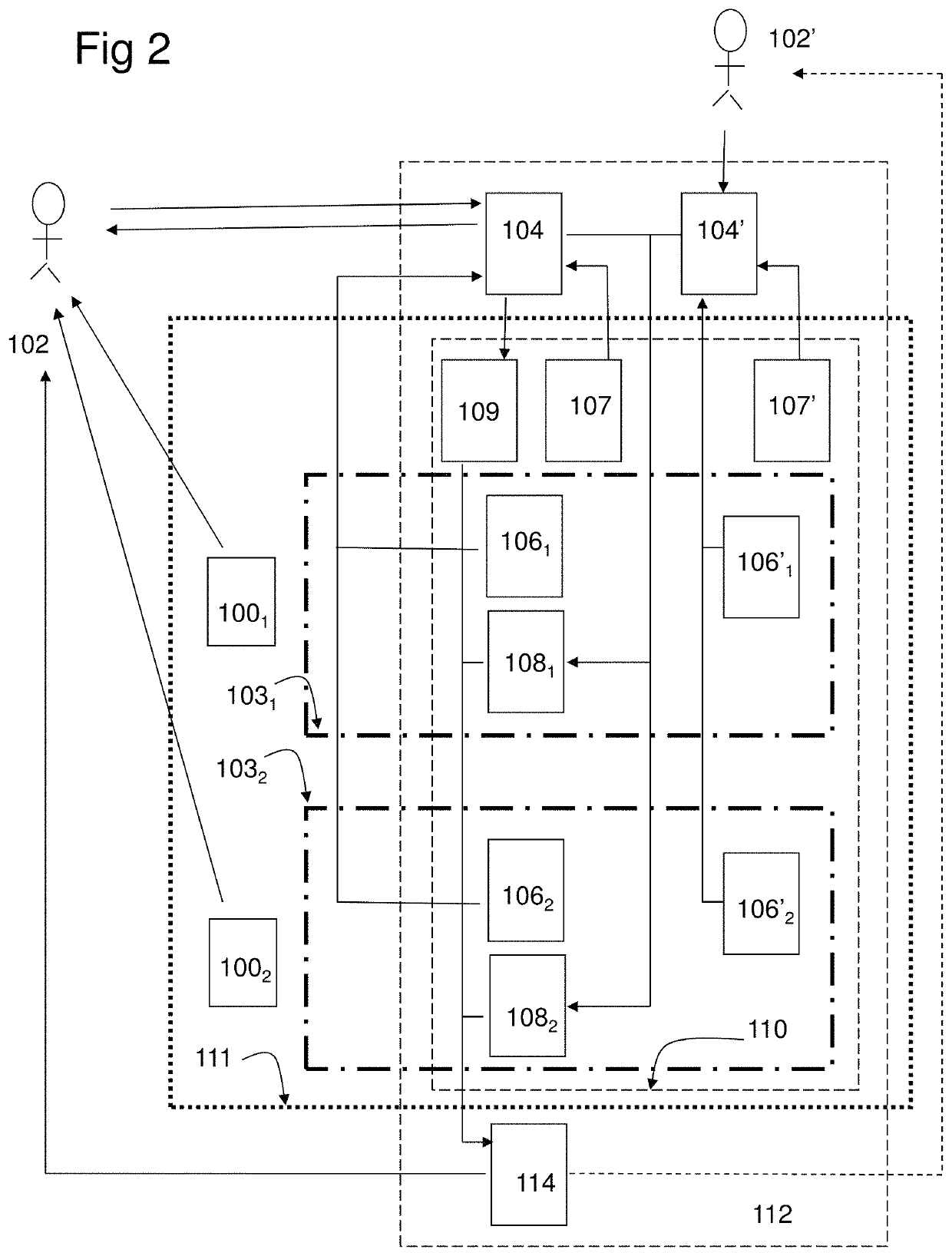
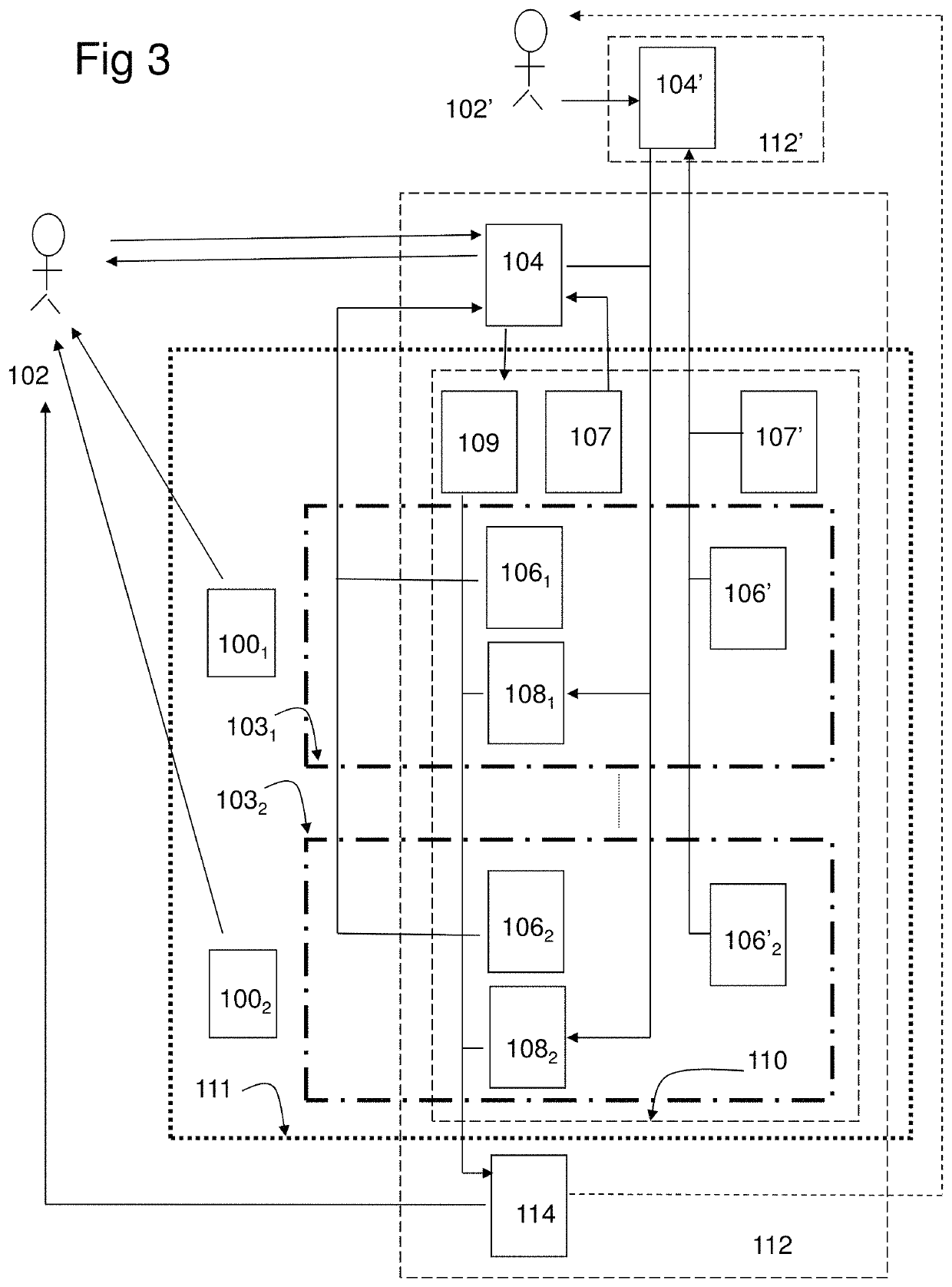
[0222]In the study the combination product, a computer program product (CPP) integrated with two pharmaceutical products (PP:s), using an adapted question-analysis-feedback model (QAFM) and two question-feedback models (QFM:s), should be evaluated versus only the two separate PP:s. The purpose is to evaluate different aspects in order to show the effect of the invention. The objective of the study should be to evaluate the clinical effect of a combination of two PP:s and a CPP, in relation to only the two PP:s. The integration in the combination product should be done through a QAFM and two QFM:s. The actual therapy area is type 1 diabetes and the effect variable should be the level of HbA1c. The actual PP:s are Apidra and Lantus. In the study the used model should consist of the following parts:[0223]A set of questions for the QAFM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com