A kind of pharmaceutical composition containing adalimumab
A technology of adalimumab and a composition, applied in the biological field, can solve problems such as the impact of biopharmaceutical safety, reduce the efficacy of biological drugs, patient death, etc., and achieve broad market application prospects, high stability, and the effect of improving stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
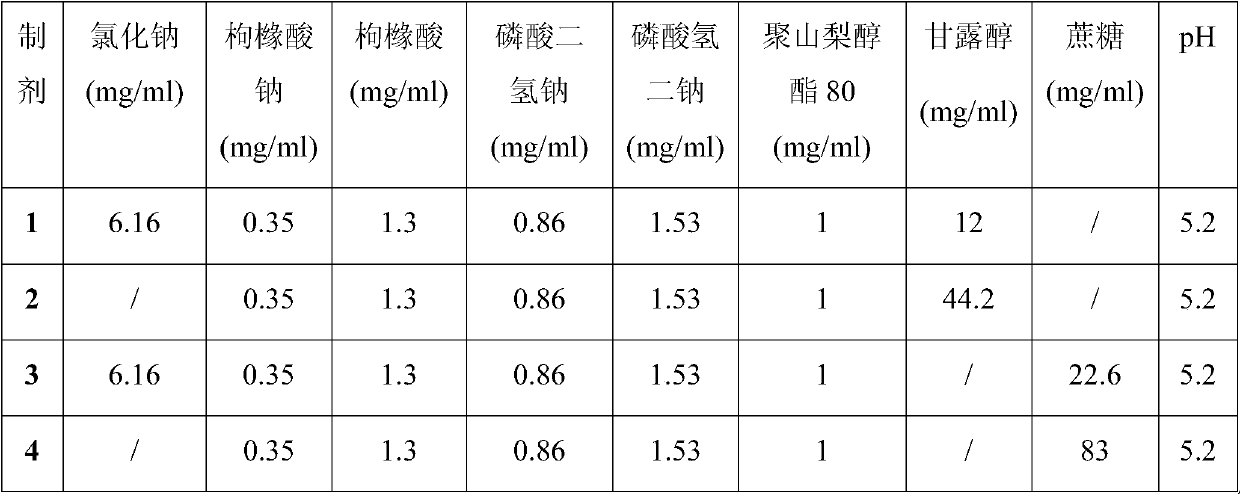
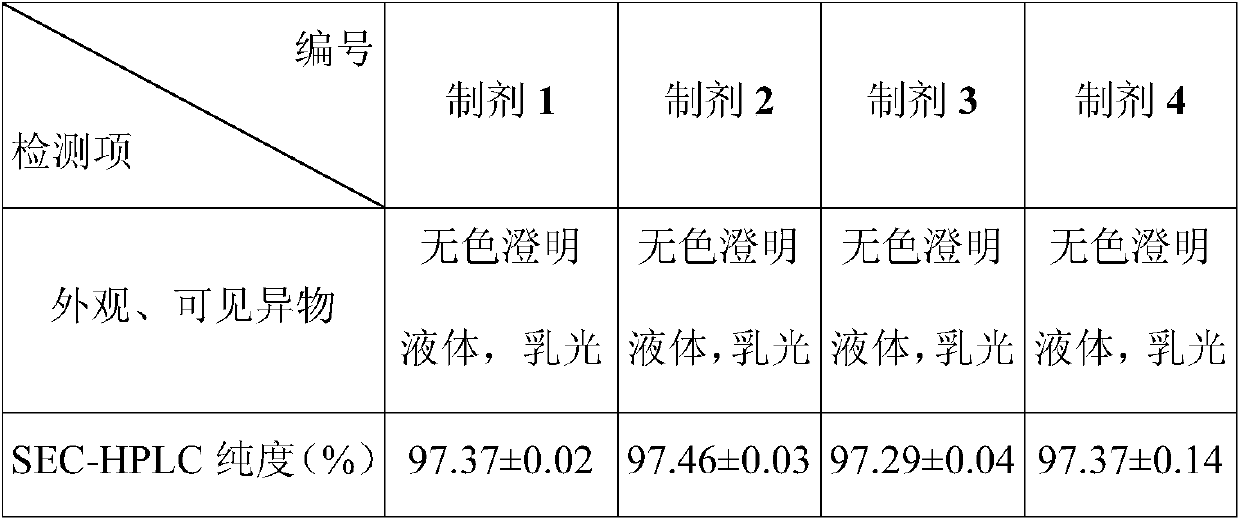
Embodiment 1
[0028] Embodiment 1 Stability Study (1)
[0029] Biopharmaceuticals will experience various destructive conditions during production, transportation, storage and use, such as high temperature, light and freeze-thaw. To be safe, they must be able to maintain structural integrity, especially higher-level structures. Aggregates and insoluble particles of biopharmaceuticals are very critical factors that cause immune responses to biopharmaceuticals. The appearance of biopharmaceuticals is also a very important indicator. Pharmacopoeias of various countries stipulate that biological drugs must be free of visible foreign matter, because the presence or absence and content of particles in biological drugs are directly related to the safety of biological drugs, and they are considered to be one of the most important factors that cause the immune response of biological drugs.
[0030] SEC-HPLC is an analytical method that uses the relative relationship between the pore size of the ge...
Embodiment 2
[0060] Embodiment 2 stability study (two)
[0061] We have designed several preparation prescriptions 5-9, combined with the experimental results of preparation 2 and preparation 4 in Example 1, compared the freeze-thaw stability of the preparations with mannitol, sucrose and trehalose at different concentrations of adalimumab Among them, these preparations do not contain sodium chloride, and the other components in the preparation are: 0.35mg / ml sodium citrate, 1.3mg / ml citric acid, 0.86mg / ml sodium dihydrogen phosphate, 1.53mg / ml Disodium hydrogen phosphate, 1 mg / ml polysorbate 80, pH value of the solution is 5.2. The prescriptions of these preparations were frozen at -20°C for 5 hours, thawed at 4°C for 10 hours, and tested by SEC-HPLC after repeated freezing and thawing 8 times. The results are shown in Table 6.
[0062] Table 6 Effects of mannitol, sucrose and trehalose on the freeze-thaw stability of formulations under different adalimumab concentrations
[0063]
...
Embodiment 3
[0065] Embodiment 3: the preparation of low concentration (1mg / ml) and high concentration (100mg / ml) adalimumab preparation of sucrose and trehalose as structural protection agent
[0066] We designed and formulated formulations 10-13 containing low (1 mg / ml) and high (100 mg / ml) concentrations of adalimumab, respectively (Table 7), for validation of sucrose and trehalose as structural protectors Ability to formulate low and high concentration adalimumab formulations. These preparations do not contain sodium chloride, other components are: 0.35mg / ml sodium citrate, 1.3mg / ml citric acid, 0.86mg / ml sodium dihydrogen phosphate, 1.53mg / ml disodium hydrogen phosphate, 1mg / ml polysorbate 80, the solution pH value is 5.2.
[0067] Table 7 Preparations containing high and low concentrations of adalimumab with sucrose and trehalose as structural protective agents
[0068]
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com