Recombinant vector for enhancing ability of displaying Fab fragment antigen binding on yeast cell surface by using endoplasmic reticulum retrieval signal sequence
A yeast cell and surface display technology, which is applied in the direction of introducing foreign genetic material using vectors, recombinant DNA technology, DNA/RNA fragments, etc., can solve the problems of weak yeast display efficiency, insufficient effective assembly and folding, etc., to promote folding and Assembly efficiency, improvement of antigen-binding ability, effect of improving antigen-binding ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
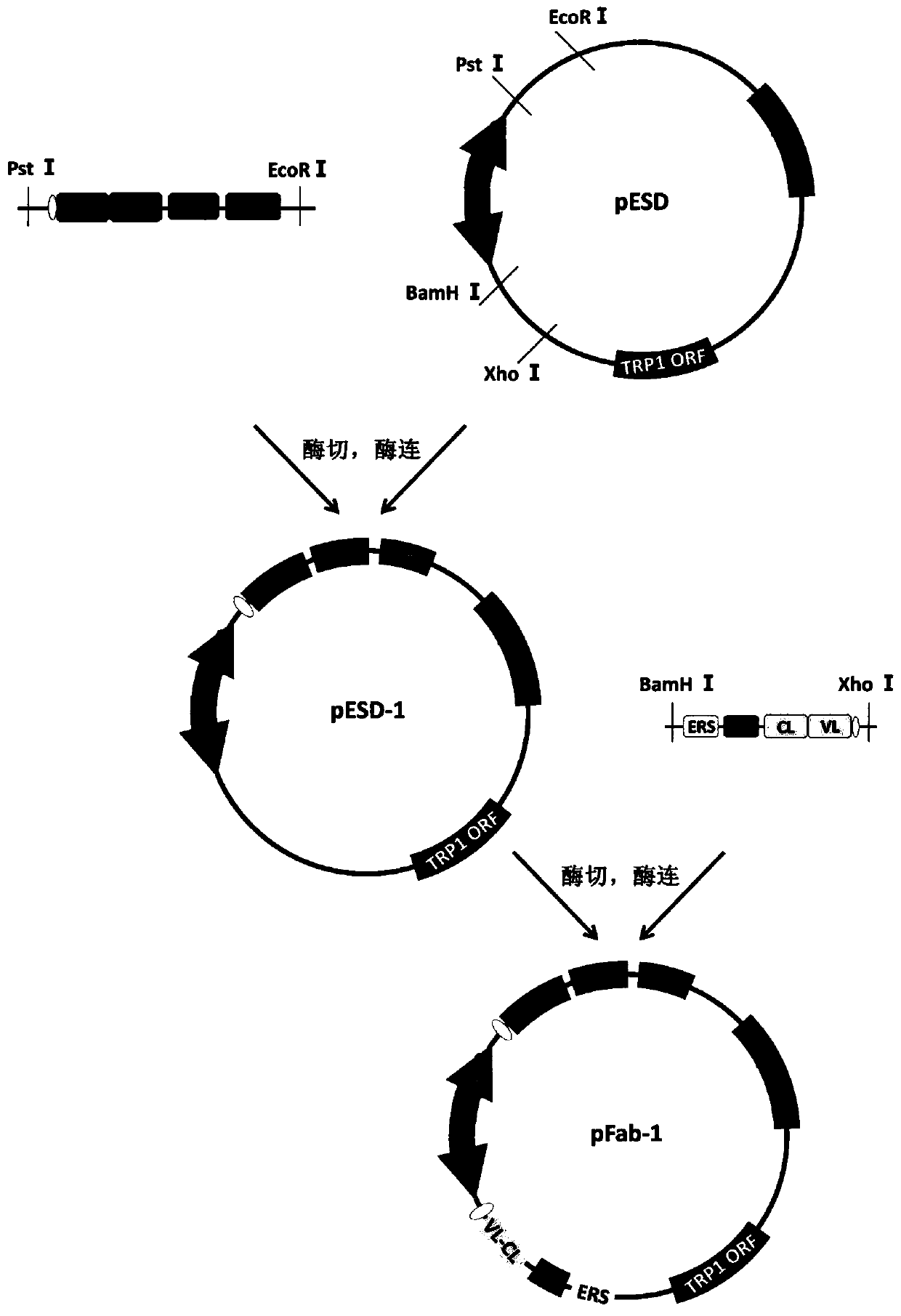
[0033] Embodiment 1: the construction of the genetically engineered bacteria containing plasmid pFab-1
[0034] The gene fragments of VH-CH1-FLAG-Aga2 and VL-CL-HA-ERS complexes of D2E7 and Infliximab were obtained by PCR cloning method;
[0035] PCR reaction system: 10×KOD buffer, 5 μl; dNTP (2.5mM), 4 μl; primer F (10 μM), 3 μl; primer R (10 μM), 3 μl; Pfu polymerase, 2 μl; template, 1 μl; add ddH 2 0 to 50 μl. PCR amplification system: 95°C, 5min; 95°C, 30s, 55°C, 30s, 72°C, 30s, 25 cycles; 72°C, 5min; 12°C, 10min;
[0036] After the PCR product of the VH-CH1-FLAG-Aga2 complex obtained above was recovered with 0.8% agarose gel, it was double-digested with restriction endonucleases PstI and EcoRI. The enzyme digestion system was: PstI, 1 μl; EcoRI , 1μl; 10×Cutsmartbuffer, 5μl; PCR recovered product, 30μl, add ddH 2 0 to 50 μl. After digestion at 37°C for 5 hours, recover with 0.8% agarose gel;
[0037] Then it was ligated with the pESD vector that had been digested wit...
Embodiment 2
[0040] Embodiment 2: Fab-induced expression of genetically engineered bacteria containing plasmid pFab-1
[0041] The yeast transformant transformed with pFab-1 plasmid in Example 1 was inoculated in YNB-CAA-Glucose liquid medium, and cultured overnight at 30°C until OD 600 3.0-4.0, change YNB-CAA-Galactose medium to induce, initial OD600 0.8, induced at 18°C for 48h.
Embodiment 3
[0042] Example 3: Fab yeast cell surface display efficiency and activity detection of genetically engineered bacteria containing plasmid pFab-1
[0043] For the yeast cells induced in Example 2, two samples were taken from each sample, one was used for the detection of Fab display efficiency, and the other was used for the detection of activity. Take 10 respectively 6 For each yeast cell, wash once with solution A, then wash once with solution B, centrifuge at 4°C, 3000rpm for 2min. One of the cells used for Fab activity detection was incubated with different concentrations of 6×His-TNF-α (expressed and purified in Escherichia coli) at 25°C for 30 min, then washed twice with solution B, and finally washed with 20 μl solution B and 0.15 Resuspend in μl fluorescent antibody Anti-6×His-iFluor 647. Another portion of cells used for Fab display efficiency detection was directly resuspended with 20 μl solution B and 0.15 μl fluorescent antibody Anti-HA-FITC. The resuspended cells...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com