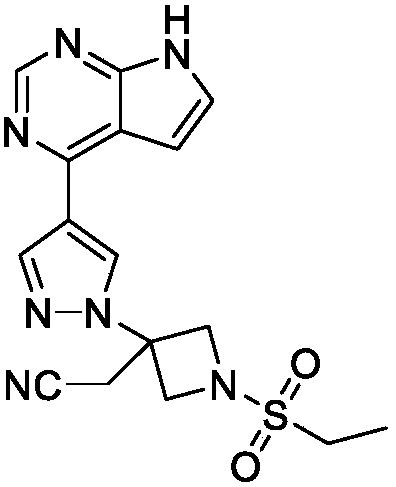
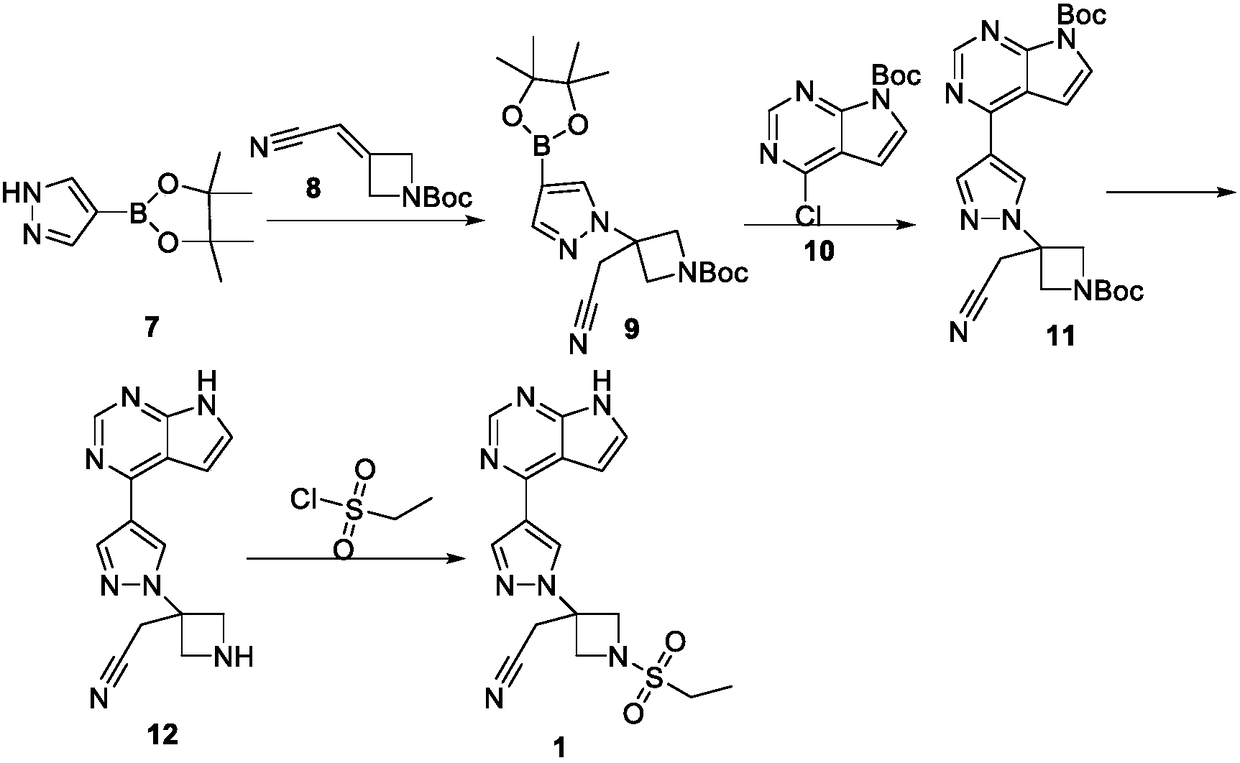
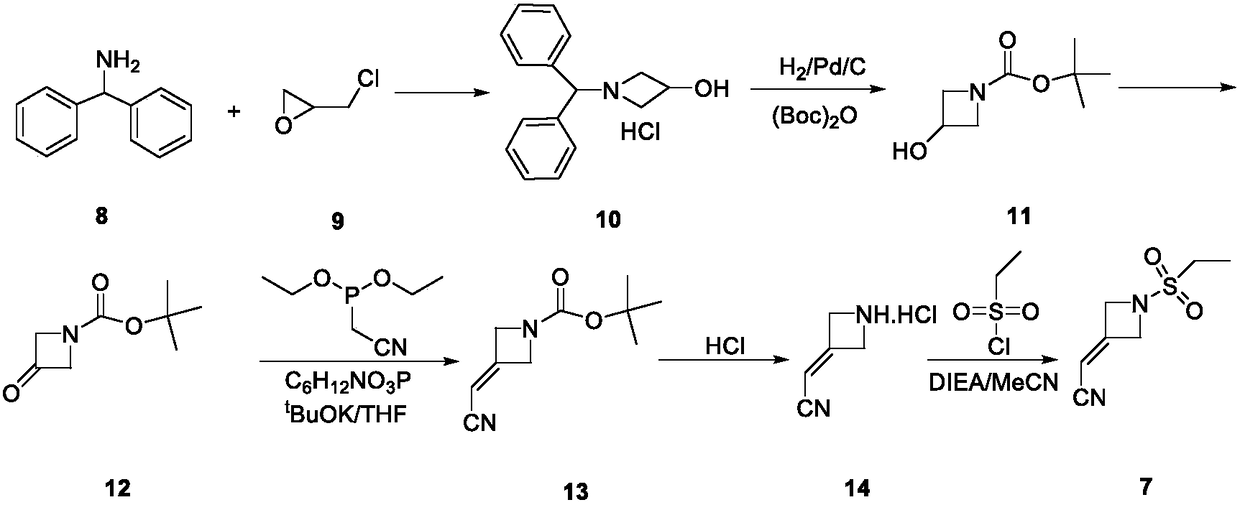
Preparation method of baricitinib
A baricitinib and compound technology, applied in the field of drug preparation, can solve the problems of unsuitability for industrial production, long reaction route, complicated operation, etc., so as to reduce the possibility of by-product generation, improve the quality and the total yield, reaction short route effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Synthesis of Compound 4
[0043] Put 15.4g (0.1mol) of compound 2, 11.4g (0.05mol) of dipotassium hydrogen phosphate, 26.2g (0.12mol) of di-tert-butyl dicarbonate, 300g of tetrahydrofuran and 70g of water into a 1L reaction bottle, stir at room temperature for 12 hours, and then control , the reaction of the raw materials was complete, the layers were separated, the aqueous layer was extracted with 200 g of tetrahydrofuran, the organic layers were combined, and the organic layers were directly used in the next step.
[0044] 5.1 g (0.1 mol) of hydrazine hydrate and 5.6 g (0.1 mol) of acrolein were added to the above system, and the mixture was refluxed for 8 hours under an oxygen atmosphere. Stop heating after the reaction of the central control raw material is complete, cool the system down to room temperature, pour the system into 100g of ice water with stirring, layer after stirring for 10min, dry and filter the organic layer, and rotary evaporate the filtrate to obt...
Embodiment 2
[0055] Synthesis of Compound 4
[0056] Put 15.4g (0.1mol) of compound 2, 11.4g (0.05mol) of dipotassium hydrogen phosphate, 31.0g (0.12mol) of fluorenylmethoxycarbonyl chloride, and 400g of dichloromethane into a 1L reaction bottle, stir at room temperature for 12 hours, and control in the central control. After the reaction was complete, filter, add 300 g of ice water to the filtrate, stir for 10 min, then separate the liquids, extract the water layer with 200 g, combine the organic layers, spin the organic layers to dry, and use the obtained system directly in the next reaction.
[0057] 10.2 g (0.2 mol) of hydrazine hydrate, 500 g of toluene and 11.2 g (0.2 mol) of acrolein were added to the above system, and the reaction was refluxed for 8 h under an oxygen atmosphere. Stop heating after the reaction of the central control raw material is complete, cool the system down to room temperature, pour the system into 100g of ice water with stirring, layer after stirring for 10mi...
Embodiment 3
[0067] Synthesis of Compound 4
[0068] Put 15.4g (0.1mol) of compound 2, 27.6g (0.2mol) of potassium carbonate, 19.1g (0.1mol) of p-toluenesulfonyl chloride, 500g of acetonitrile into a 1L reaction bottle, stir at room temperature for 8 hours, and then control in the middle, the raw materials react completely, filter, The filtrate was spin-dried under reduced pressure and used directly in the next step.
[0069] 20.4 g (0.4 mol) of hydrazine hydrate, 450 g of o-dichlorobenzene and 22.4 g (0.4 mol) of acrolein were added to the above system, and the reaction was refluxed under an oxygen atmosphere for 4 h. Stop heating after the reaction of the central control raw material is complete, cool the system down to room temperature, pour the system into 100g of ice water with stirring, layer after stirring for 10min, dry and filter the organic layer, and rotary evaporate the filtrate to obtain the crude sticky substance. About 27.7 g of compound 4 was obtained by recrystallization ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com