Method for separating and determining baricitinib bulk drug impurities by using HPLC
A technology for raw materials and impurities, which is applied in the field of analysis and determination of baricitinib raw materials, can solve the problems of lack of rapid, simple and accurate analysis and detection of baricitinib raw materials, and achieves high sensitivity and high precision. , the effect of strong accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Chromatographic column: ChromCore C18(2)5μm250×4.6mm
[0059] Mobile phase A: water
[0060] Mobile Phase B: Acetonitrile
[0061] Column temperature: 35℃
[0062] Flow rate: 1.0ml / min
[0063] Detection wavelength: 224nm
[0064] Injection volume: 10μl
[0065] The conditions of gradient elution are:
[0066] Time, minutes Mobile phase A, volume% Mobile phase B, volume% 09010 39010 452080 469010 559010
[0067] Sample preparation:
[0068] Thinner: Acetonitrile
[0069] Blank solution: thinner
[0070] Reference substance solution: Take about 20mg of Baritinib working reference substance, accurately weigh it, place it in a 20ml measuring bottle, add diluent to dissolve and dilute to the mark, shake well, accurately measure 1ml to 10ml, add diluent to dissolve and dilute To the mark, shake well, and then accurately measure 1ml to 10ml, add diluent to dissolve and dilute to the mark, shake, and then accurately measure 1ml to 10ml, add the diluent to dissolve and dilute to the mark, ...
Embodiment 2
[0086] Chromatographic column: ChromCoreC18(2)5μm250×4.6mm
[0087] Mobile phase A: water
[0088] Mobile Phase B: Acetonitrile
[0089] Column temperature: 35℃
[0090] Flow rate: 1.0ml / min
[0091] Detection wavelength: 224nm
[0092] Injection volume: 10μl
[0093] The conditions of gradient elution are:
[0094] Time, minutes Mobile phase A, volume% Mobile phase B, volume% 0905 3905 452080 46905 55905
[0095] Sample preparation:
[0096] The same sample preparation as in Example 1.
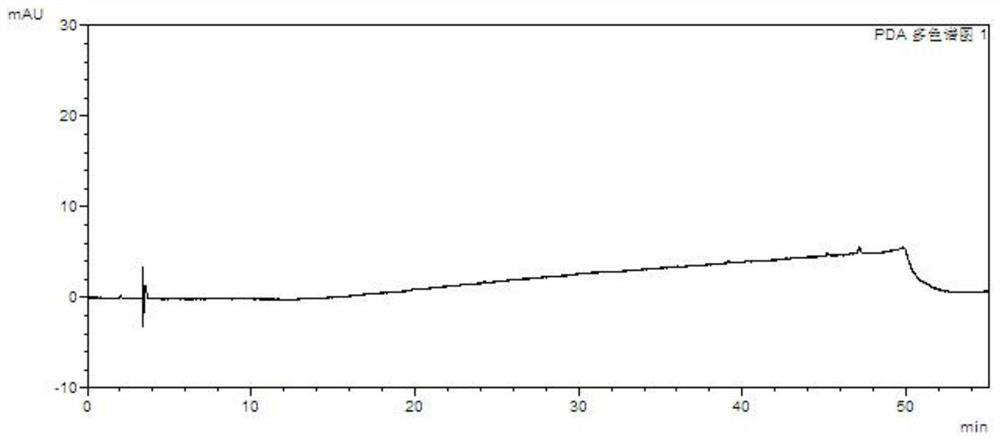
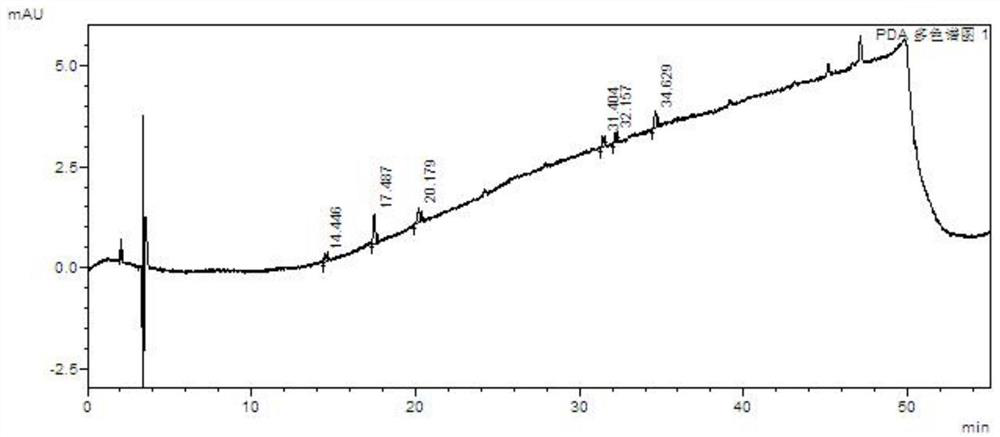
[0097] Take the above solutions separately, perform high performance liquid chromatography analysis under the above chromatographic conditions, record the chromatograms, and see the results Figure 7 , Attached Figure 8 , Attached Picture 9 , Attached Picture 10 , Attached Picture 11 , Attached Picture 12 .
[0098] in conclusion: Figure 7 Indicates that the blank does not interfere with the impurity inspection; Figure 8 It shows that the detection limits of baritinib and impurity A, impurity B, im...
Embodiment 3
[0102] Chromatographic column: ChromCoreC18(2)5μm250×4.6mm
[0103] Mobile phase A: water
[0104] Mobile Phase B: Acetonitrile
[0105] Column temperature: 35℃
[0106] Flow rate: 1.0ml / min
[0107] Detection wavelength: 224nm
[0108] Injection volume: 10μl
[0109] The conditions of gradient elution are:
[0110] Time, minutes Mobile phase A, volume% Mobile phase B, volume% 09010 39010 452575 469010 559010
[0111] Sample preparation:
[0112] The same sample preparation as in Example 1.
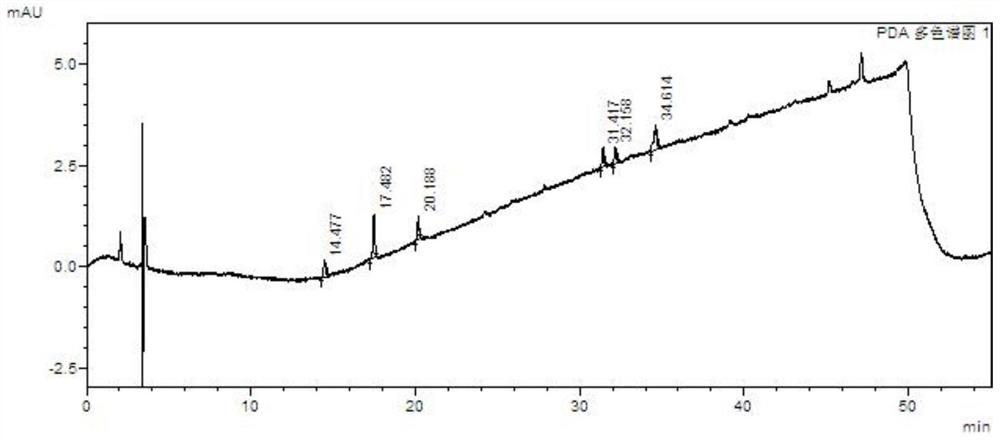
[0113] Take the above solutions separately, perform high performance liquid chromatography analysis under the above chromatographic conditions, record the chromatograms, and see the results Figure 13 , Attached Figure 14 , Attached Figure 15 , Attached Figure 16 , Attached Figure 17 , Attached Figure 18 .
[0114] in conclusion: Figure 13 Indicates that the blank does not interfere with the impurity inspection; Figure 14 It shows that the detection limits of baritinib and impurity A, impurity...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com