Drug composition containing baricitinib and preparation method and application of drug composition
A baricitinib and composition technology, which is applied in the field of pharmaceutical compositions for improving drug dissolution, can solve the problems of poor process repeatability, unfavorable scale-up production, and high process costs, and achieves good mixing uniformity, not easy to decompose, and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
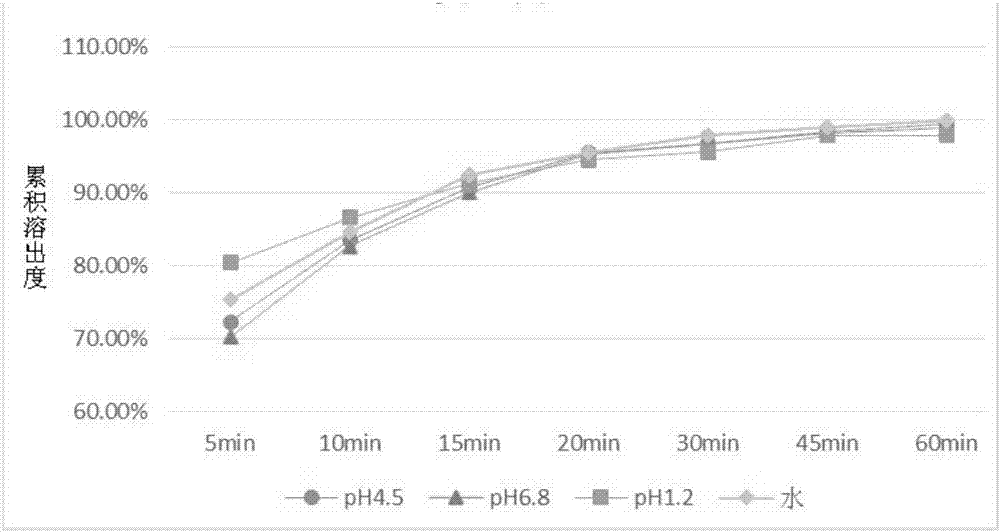
[0068]The preparation method of the baricitinib-containing pharmaceutical composition of the present invention is specifically described below. The present invention mainly discloses a process for preparing the above-mentioned baricitinib-containing pharmaceutical composition into tablets, which mainly involves the steps of mixing, granulating and tableting. Among them, when the dosage of the main drug (such as baricitinib) is small, the present invention improves the material mixing mode of the main drug and the auxiliary material, so as to ensure the uniformity of mixing and further ensure the dissolution efficiency of the main drug. For example, when mixing materials, the main drug can be mixed with the filler first, and then mixed with other auxiliary materials; after the auxiliary materials are evenly mixed, the main drug can be added for mixing.
[0069] According to an exemplary embodiment of the present invention, the specific preparation method is: uniformly mix the f...
Embodiment 1
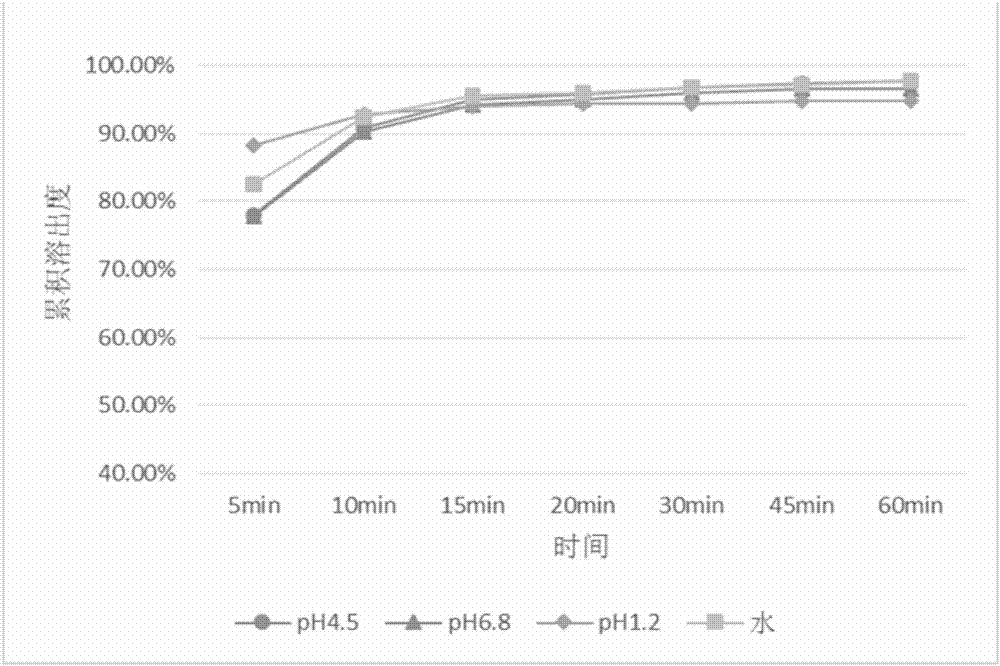
[0086] Embodiment 1: the preparation of the pharmaceutical composition (coated tablet) containing baricitinib
[0087] This embodiment prepares the coated tablet containing baricitinib, adopts the tablet core prescription containing baricitinib listed in Table 1, the weight gain of Opadry film coating is 2% of the tablet core weight, baricitinib The particle size of ricitinib raw material is 0.075mm-0.048mm.
[0088] Table 1 Tablet core prescription of pharmaceutical composition containing baricitinib
[0089] Main ingredients and excipients
Mass percentage content (wt%)
1
55
40
3
1
Chip sum
100
[0090] The specific preparation method is as follows: weigh microcrystalline cellulose, mannitol and croscarmellose sodium according to the tablet core prescription in Table 1, and premix to obtain mixed excipients; di...
Embodiment 2
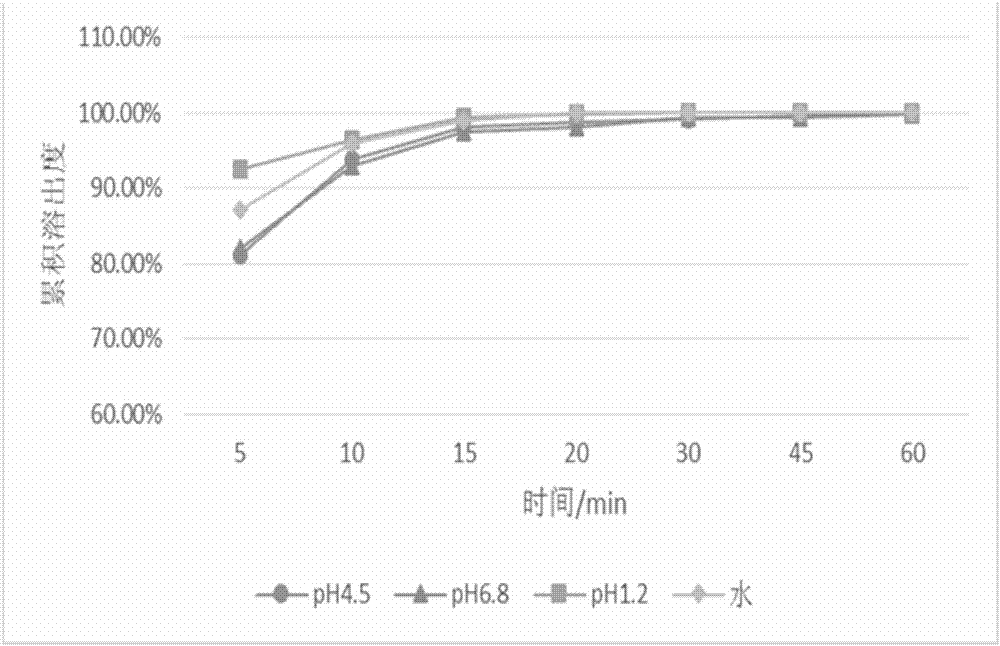
[0096] Embodiment 2: Preparation of the pharmaceutical composition containing baricitinib
[0097] The prescription of the pharmaceutical composition containing baricitinib in the embodiment 2 of table 3
[0098] Main ingredients and excipients
Mass percentage content (wt%)
4
46
46
3
1
sum
100
[0099] The data in Table 3 shows the prescription of the pharmaceutical composition containing baricitinib used in this example, wherein the particle size of the baricitinib bulk drug is less than 0.048um.
[0100] The specific preparation method is as follows: weighing microcrystalline cellulose, mannitol and cross-linked carmellose sodium according to the prescription, stirring and mixing uniformly to obtain mixed auxiliary materials. Disperse baricitinib in pure water to prepare a granulation liquid; spray the granula...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com