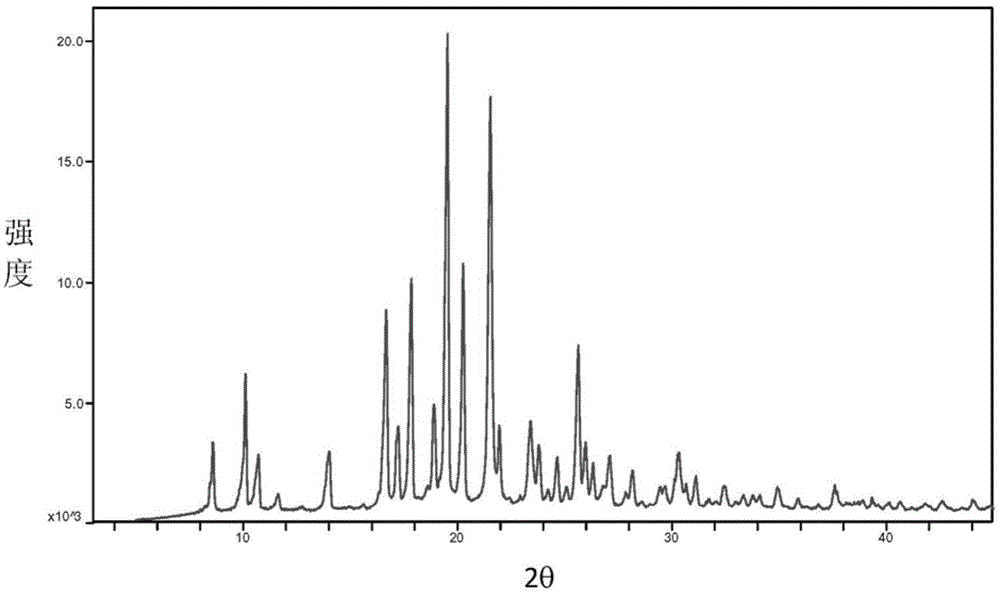
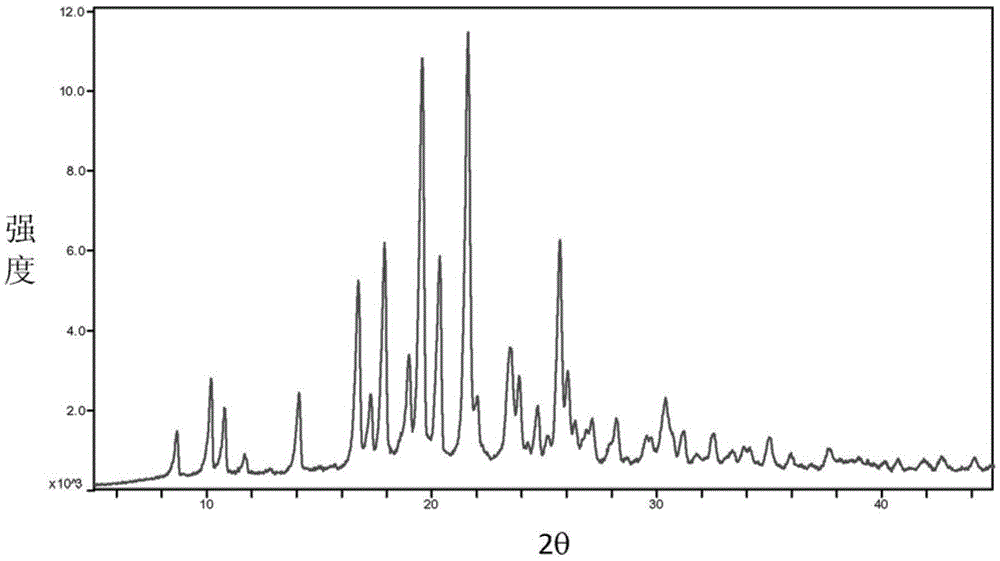
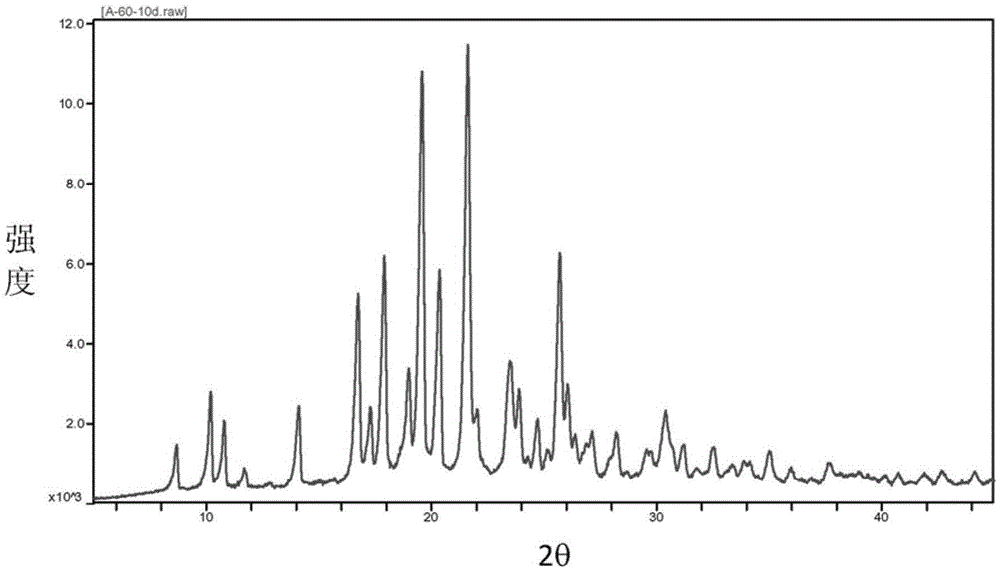
Baricitinib trifluoroacetate crystal forms A and B and preparation method thereof
A technology of trifluoroacetate and crystal form of baricitinib, applied in the field of crystal form A and crystal form B of baricitinib trifluoroacetate and its preparation, can solve the problem of affecting bioavailability, baricitinib The stability of nitrifluoroacetate needs to be improved to achieve the effect of high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 Preparation of crystal form A of baricitinib trifluoroacetate
[0060] Add 400 mg of baricitinib trifluoroacetate semi-crystals into 4 mL of acetone to prepare a solution at 50 ° C, and the solution is rapidly cooled to 15 ° C under ultrasonic conditions, then filtered, dried to obtain a solid, and the weight of the solid is weighed is 106mg.
Embodiment 2
[0061] Example 2 Preparation of crystal form A of baricitinib trifluoroacetate
[0062] Add 400 mg of baricitinib trifluoroacetate semi-crystals into 3.3 mL of acetone to prepare a solution at 54°C. The solution is rapidly cooled to 0°C under ultrasonic conditions, filtered, and dried to obtain a solid. Weigh the solid The weight is 113mg.
Embodiment 3
[0063] Example 3 Preparation of crystal form A of baricitinib trifluoroacetate
[0064] Add 400 mg of baricitinib trifluoroacetate semi-crystals into 5 mL of acetone to prepare a solution at 52 °C, and then quickly cool the solution to 20 °C under ultrasonic conditions, then filter, dry to obtain a solid, and weigh the weight of the solid is 97mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com